Summary
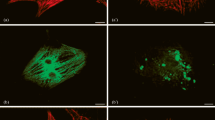
Neonatal rat cardiomyocytes were cultured on extracellular matrix components laminin and collagens I+III to examine effects of extracellular matrix on the assembly of cytoskeletal proteins during myofibrillogenesis. Myofibril assembly was visualized by immunofluorescence of marker proteins for myofibrils (f-actin for I bands and α-actinin for Z bands), focal adhesions (vinculin), and transmembrane extracellular matrix receptors (β1 integrin) as cells spread for various times in culture. By 4 h in culture, f-actin appeared organized into nonstriated stress-fiber-like structures while α-actinin, vinculin and β1 integrin were localized in small streaks and beads. Subsequently, striated patterns were observed sequentially in the intracellular cytoskeletal components α-actinin, vinculin, f-actin, and then in the transmembrane β1 integrin receptor. These data support an earlier model for sarcomerogenesis in which stress-fiber-like structures serve as initial scaffolds upon which α-actinin and then vinculin-containing costameres are assembled. This sequential and temporal assembly was the same on both laminin and collagens I+III. A quantitative difference, however, was apparent on the 2 matrices. There was an increased appearance on collagens I+III of rosettes (also called podosomes or cortical actin-containing bodies in other cells) which consisted of an f-actin core surrounded by α-actinin, vinculin and β1 integrin rims. The increased incidence of rosettes in neonatal myocytes on collagens I+III suggests that these cytoskeletal complexes are involved in recognition and interaction with extracellular matrix components.
Similar content being viewed by others
References
Abercrombie M, Heaysman JEM, Pegrum SM (1971) The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res 67:359–367
Atherton BT, Meyer DM, Simpson DG (1986) Assembly and remodelling of myofibrils and intercalated discs in cultured neonatal rat heart cells. J Cell Sci 86:233–248
Blikstad I, Carlsson L (1982) On the dynamics of the microfilament system in HeLa cells. J Cell Biol 93:122–128
Blobel G, Dobberstein B (1975) Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol 67:835–851
Borg TK, Terracio L (1988) Cellular adhesion to artificial substrates and long term culture of adult cardiac myocytes: In: Clark WA, Decker RS, Borg TK (eds) Biology of isolated adult cardiac myocytes. Elsevier, New York, pp 14–24
Borg TK, Terracio L (1989) Interaction of the extracellular matrix with cardiac myocytes during development and disease. In: Kinne R, Stolte H (eds) Issues in biomedicine: cardiac myocyteconnective tissue interactions in health and disease. Karger, Basel, pp 113–129
Borg TK, Rubin K, Lundgren E, Borg K, Öbrink B (1984) Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol 104:86–96
Borg TK, Xuehui M, Hilenski L, Vinson N, Terracio L (1990) The role of the extracellular matrix on myofibrillogenesis in vitro. In: Clark EB, Takao A (eds) Developmental cardiology: morphogenesis and function. Futura, Mt Kisco, NY, pp 175–190
Brugge JS, Erikson RL (1977) Identification of transformationspecific antigen induced by an avian sarcoma virus. Nature 269:346–348
Buck CA, Horwitz AF (1987) Cell surface receptors for extracellular matrix molecules. Ann Rev Cell Biol 3:179–205
Burnette WN (1981) Western blotting:electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203
Burridge K (1986) Substrate adhesions in normal and transformed fibroblasts:organization and regulation of cytoskeletal, membrane and extracellular matrix components at focal contacts. Cancer Rev 4:18–78
Carley WW, Barak LS, Webb WW (1981) F-actin aggregates in transformed cells. J Cell Biol 90:797–802
Caufield JB, Borg TK (1979) The collagen network of the heart. Lab Invest 40:364–372
Chen W-T (1989) Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool 251:167–185
Chen W-T, Singer SJ (1982) Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol 95:205–222
Chen W-T, Olden K, Bernard BA, Chu F-F (1984) Expression of transformation-associated protease(s) that degrade fibronectin at cell contact sites. J Cell Biol 98:1546–1555
Chen W-T, Hasegawa E, Hasegawa T, Weinstock C, Yamada KM (1985a) Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol 100:1103–1114
Chen W-T, Chen J-M, Parsons SJ, Parsons JT (1985b) Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature 316:156–158
Colley NJ, Tokuyasu KT, Singer SJ (1990) The early expression of myofibrillar proteins in round postmitotic myoblasts of embryonic skeletal muscle. J Cell Sci 95:11–22
Croop J, Holtzer H (1975) Response of myogenic and fibrogenic cells to cytochalasin B and to colcemid. 1. Light microscope observations. J Cell Biol 65:271–285
Damsky CH, Knudsen KA, Bradley D, Buck CA, Horwitz AF (1985) Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol 100:1528–1539
David-Pfeuty T, Singer SJ (1980) Altered distribution of the cytoskeletal proteins vinculin and α-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci USA 77:6687–6691
Dejana E, Colella S, Conforti G, Abbadini M, Gaboli M, Marchisio PC (1988) Fibronectin and vitronectin regulate the organization of their respective arg-gly-asp adhesion receptors in cultured human endothelial cells. J Cell Biol 107:1215–1223
Denning GM, Kim IS, Fulton AB (1988) Shedding of cytoplasmic actins by developing muscle cells. J Cell Sci 89:273–282
Dlugosz AA, Antin PB, Nachmias VT, Holtzer H (1984) The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol 99:2268–2278
Duband J-L, Nuckolls GH, Ishihara A, Hasegawa T, Yamada KM, Thiery JP, Jacobson K (1988) Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol 107:1385–1396
Fath KR, Edgell C-JS, Burridge K (1989) The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci 92:67–75
Fischman DA (1986) Myofibrillogenesis and the morphogenesis of skeletal muscle. In: Engel AG, Banker BQ (eds) Myology: basic and clinical. McGraw-Hill, New York, pp 5–37
Fürst DO, Osborn M, Weber K (1989) Myogenesis in the mouse embryo: differential onset of expression of myogenic protein and the involvement of titin in myofibril assembly. J Cell Biol 109:517–527
Geiger B, Avnur Z, Kreis TE, Schlessinger J (1984) The dynamics of cytoskeletal organization in areas of cell contact. Cell Muscle Motil 5:195–234
Gullberg D, Terracio L, Borg TK, Rubin K (1989) Identification of integrin-like matrix receptors with affinity for interstitial collagens. J Biol Chem 264:12686–12694
Handel SE, Wang S-M, Greaser ML, Schultz E, Bulinksi JC, Lessard JL (1989) Skeletal muscle myofibrillogenesis as revealed with a monoclonal antibody to titin in combination with detection of the α- and gamma-isoforms of actin. Dev Biol 132:35–44
Hilenski LL, Terracio L, Sawyer R, Borg TK (1989) Effects of extracellular matrix on cytoskeletal and myofibrillar organization in vitro. Scanning Microsc 3:535–548
Horwitz A, Duggan K, Buck C, Berkerle MC, Burridge K (1986) Interaction of plasma membrane fibronectin receptor with talin: a transmembrane linkage. Nature 320:531–533
Hynes RO (1987) Integrins: a family of cell surface receptors. Cell 48:549–554
Isobe Y, Warner FD, Lemanski LF (1988) Three-dimensional immunogold localization of α-actinin within the cytoskeletal networks of cultured cardiac muscle and nonmuscle cells. Proc Natl Acad Sci USA 85:6758–6762
Kaufmann R, Frösch D, Westphal D, Weber L, Klein CE (1989) Integrin VLA-3: ultrastructural localization at cell-cell contact sites of human cell cultures. J Cell Biol 109:1807–1815
Lampugnani MG, Dejana E, Abbadini M, Marchisio PC (1988) Organization of vitronectin and fibronectin receptors in the endothelial cell membrane. In: Rousset BAF (ed) Colloque INSERM, vol 171 Libbey, London, pp 133–138
Legato MJ (1972) Ultrastructural characteristics of the rat ventricular cell grown in tissue culture, with special reference to sarcomerogenesis. J Mol Cell Cardiol 4:299–317
Levinson AD, Oppermann H, Levintow L, Varmus HE, Bishop JM (1978) Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell 15:561–572
Lin Z, Eshleman J, Grund C, Fischman DA, Masaki T, Franke WW, Holtzer H (1989a) Differential response of myofibrillar and cytoskeletal proteins in cells treated with phorbol myristate acetate. J Cell Biol 108:1079–1091
Lin Z, Holtzer S, Schultheiss T, Murray J, Masaki T, Fischman DA, Holtzer H (1989b) Polygons and adhesion plaques and the disassembly and assembly of myofibrils in cardiac myocytes. J Cell Biol 108:2355–2367
Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G (1987) Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res 169:202–214
Marchisio PC, Bergui L, Corbascio GC, Cremona O, D'Urso N, Schena M, Tesio L, Caligaris-Cappio F (1988) Vinculin, talin, and integrins are localized in specific adhesion sites of malignant B lymphocytes. Blood 72:830–833
Markwald RR (1973) Distribution and relationship of precursor Z material to organizing myofibrillar bundles in embryonic rat and hamster ventricular myocytes. J Mol Cell Cardiol 5:341–350
Otey CA, Pavalko FM, Burridge K (1989) Integrin-binding proteins from chicken embryo fibroblasts. J Cell Biol 109:190a
Pardo JV, Siliciano JD, Craig SW (1983) A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA 80:1008–1012
Peng HB, Wolosewick JJ, Cheng P-C (1981) The development of myofibrils in cultured muscle cells: a whole-mount and thinsection electron microscope study. Dev Biol 88:121–136
Ruoslahti E, Pierschbacher MD (1987) New perspectives in cell adhesion: RGD and integrins. Science 238:491–497
Schultheiss T, Lin Z, Lu M-H, Murray J, Fischman DA, Weber K, Masaki T, Imamura M, Holtzer H (1990) Differential distribution of subsets of myofibrillar proteins in cardiac nonstriated and striated myofibrils. J Biol 110:1159–1172
Sefton BM, Beemon K, Hunter T (1978) Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol 28:957–971
Shimada Y, Fischman DA (1975) Cardiac cell aggregation by scanning electron microscopy. In: Lieberman M, Sano T (eds) Developmental and physiological correlates of cardiac muscle. Raven Press, New York, pp 81–101
Shimada Y, Komiyama M, Terai M, Maruyama K (1990) Early phases of myofibril assembly in embryonic chick cardiac myocytes in vitro. In: Clark EB, Takao A (eds) Developmental cardiology: morphogenesis and function. Futura, Mt Kisco, New York, pp 63–77
Siddiqui MAQ, Kumar CC (1987) Molecular genetics and control of contractile proteins. In: Spry CJF (ed) Immunology and molecular biology of cardiovascular diseases. MTP Press, Lancaster, pp 3–20
Singer II (1979) The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16:675–685
Singer II, Scott S, Kawka DW, Kazazis DM, Gailit J, Ruoslahti E (1988) Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol 106:2171–2182
Terai M, Komiyama M, Shimada Y (1989) Myofibril assembly is linked with vinculin, α-actinin and cell substrate contacts in embryonic cardiac myocytes in vitro. Cell Motil Cytoskeleton 12:185–194
Terracio L, Gullberg D, Rubin K, Craig S, Borg TK (1989) Expression of collagen adhesion proteins and their association with the cytoskeleton in cardiac myocytes. Anat Rec 223:62–71
Terracio L, Simpson DG, Hilenski L, Carver W, Decker RS, Vinson N, Borg TK (1990) Distribution of vinculin in the Z-disk of striated muscle: analysis by laser scanning confocal microscopy. J Cell Physiol 145:78–87
Timpl R, Rohde H, Robey PG, Rennard SI, Foidart J-M, Martin GR (1979) Laminin — a glycoprotein from basement membranes. J Biol Chem 254:9933–9937
Tokuyasu KT, Maher PA (1987a) Immunocytochemical studies of cardiac myofibrillogenesis in early chick embryos. I. Presence of immunofluorescent titin spots in premyofibril stages. J Cell Biol 105:2781–2793
Tokuyasu KT, Maher PA (1987b) Immunocytochemical studies of cardiac myofibrillogenesis in early chick embryos. II. Generation of α-actinin dots within titin spots at the time of the first myofibril formation. J Cell Biol 105:2795–2801
Turner CE, Glenney JR Jr, Burridge K (1990) Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol 111:1059–1068
Volk T, Fessler LI, Fessler JH (1990) A role for integrin in the formation of sarcomeric cytoarchitecture. Cell 63:525–536
Wang K (1985) Sarcomere-associated cytoskeletal lattices in striated muscle: review and hypothesis. In: Shay JW (ed) Cell and muscle motility, vol 6. Plenum Press, New York, pp 315–369
Wang K, McClure J, Tu A (1979) Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci USA 76:3698–3702
Wang S-M, Greaser ML, Schultz E, Bulinski JC, Lin JJ-C, Lessard JL (1988) Studies on cardiac myofibrillogenesis with antibodies to titin, actin, tropomyosin, and myosin. J Cell Biol 107:1075–1083
Yin HL, Stossel TP (1979) Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature 281:583–586
Ylänne J, Virtanen I (1989) The Mr 140000 fibronectin receptor complex in normal and virus-transformed human fibroblasts and in fibrosarcoma cells: identical localization and function. Int J Cancer 43:1126–1136
Zambonin-Zallone A, Teti A, Grano M, Rubinacci A, Abbadini M, Gaboli M, Marchisio PC (1989) Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a β1 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp Cell Res 182:645–652
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hilenski, L.L., Terracio, L. & Borg, T.K. Myofibrillar and cytoskeletal assembly in neonatal rat cardiac myocytes cultured on laminin and collagen. Cell Tissue Res 264, 577–587 (1991). https://doi.org/10.1007/BF00319047
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319047