Summary
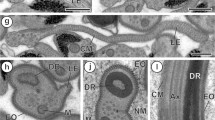
Orthoptera spermatids and spermatozoa from two species of Tettigoniidae and from one of Acrididae were analysed by means of the fluorescent lectins, concanavalin A or wheat-germ agglutinin, with the aim of finding α-D mannose· α-D glucose· β N-acetylglucosamine and sialic acid sugar residues in their plasmamembrane glycoproteins. Labelling with lectins shows remarkable changes occurring in the plasma membrane during spermiogenesis. In early spermatids, the whole cell surface is labelled, but in mature spermatids and spermatozoa, a noticeable fluorescence is restricted to the membrane that covers the acrosome. The end piece of the tail of Acrididae spermatids and spermatozoa fluoresces after wheat-germ agglutinin labelling. The intense labelling of acrosomal area is independent of acrosomal size and shape, as shown by the marked differences observed in the acrosomes of Tettigoniidae compared with Acrididae: in the former, the acrosome is a well-developed structure with an arrow-like shape, but in Acrididae, the acrosome resembles a small vesicle in the anterior tip of the cell. The large amount of some sugar residues in the plasma membrane covering the acrosome is discussed in relation to the features observed in other species, and also in connection with the physiology of the male gamete prior or during fertilization.
Similar content being viewed by others
References
Ahuja KK (1982) Fertilization studies in the hamster: the role of cell surface carbohydrates. Exp Cell Res 140:353–362
Ahuja KK (1985) Carbohydrate determinants involved in mammalian fertilization. Am J Anat 174:207–223
Aketa K (1975) Physiological studies on the sperm surface component responsible for sperm-egg bonding in sea urchin fertilization. II. Effect of concanavalin A on the fertilizing capacity of sperm. Exp Cell Res 90:56–62
Baccetti B (1987) Spermatozoa and phylogeny in orthopteroid insects. In: Baccetti B (ed) Evolutionary biology of orthoperoid insects. Ellis Hoorwood, London, pp 12–112
Baccetti B, Afzelius BA (1976) The biology of the sperm cell. Karger, New York
Baccetti B, Bagliardi E, Rosati F (1971a) The spermatozoon of Arthropoda. XIII. The cell surface. J Ultrastruct Res 35:582–605
Baccetti B, Rosati F, Selmi G (1971b) The spermatozoon of Arthropoda. XVI. The acrosome of Orthoptera. J Submicrose Cytol 3:319–337
Barnum SR, Brown GG (1983) Effect of lectins and sugars on primary sperm attachment in the horseshoe-crab Limulus polyphemus. Dev Biol 95:352–359
Bedford JM (1979) Evolution of the sperm maturation and sperm storage functions in the epididymis. In: Fawcett DW, Bedford JM (eds) The spermatozoon. Urban and Schwarzenberg, Baltimore, pp 7–21
Boldt J, Howe AM, Parkerson JB, Gunter LE, Kuehn E (1989) Carbohydrate involvement in sperm-egg fusion in mice. Biol Reprod 40:887–896
Casazza G, De Santis R, Pinto MR (1988) Plasma membrane glycoproteins of Ciona intestinalis spermatozoa that interact with the egg. Dev Growth Differ 30:147–158
Degrugillier ME, Leopold RA (1976) Ultrastructure of sperm penetration of house fly eggs. J Ultrastruct Res 56:312–325
Eddy EM (1988) The spermatozoon. In: Knobil E, Nejll JD (eds) The physiology of reproduction, vol I. Raven Press, New York, pp 27–68
Esponda P, Bedford JM (1987) Post-testicular change in the reptile sperm surface with particular reference to the snake, Natrix fasciata. J Exp Zool 241:123–132
Gordon M, Dandekar PV, Bartoszewicz (1975) The surfce coat of epididymal, ejaculated and capacitated sperm. J Ultrastruct Res 50:199–207
Guerra R, Carballada R, Esponda P (1990) A comparative analysis of the neck region in spermatids and spermatozoa of some orthopteran insects. J Morphol 204:313–321
Johnson GD, Nogueira-Araujo GMC (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods 43:349–350
Koehler JK (1981) Lectins as probes of the spermatozoon surface. Arch Androl 6:197–217
Lambert H, Van Le A (1984) Possible involvement of a sialyated component of sperm plasma membrane in sperm-zona interaction in the mouse. Gamete Res 10:153–163
Mazzini M (1987) An overview of egg structure in orthopteroid insects. In: Baccetti B (ed) Evolutionary biology of orthopteroid insects. Ellis Horwood, London, pp 358–372
Millette CF (1979) Appearance and partitioning of plasma membrane antigens during mouse spermatogenesis. In: Fawcett DW, Bedford JM (eds) The spermatozoon. Urban and Schwarzenberg, Baltimore, pp 177–186
Milette CF, Bellvé A (1977) Temporal expression of membrane antigens during mouse spermatogenesis. J Cell Biol 74:86–97
Milette CF, Scott BK (1984) Identification of spermatogenic cell plasma membrane glycoproteins by two-dimensional and lectin blotting. J Cell Sci 65:233–248
Miya K (1984) Early embryogenesis of Bombyx mori. In: Akai H, King RC (eds) Insect ultrastructure, vol II. Plenum Press, New York, pp 49–73
Nicolson GL (1974) The interaction of lectins with animal cell surfaces. Int Rev Cytol 39:89–190
Nicolson GL, Usui N, Yanagimachi R, Yanagimachi H, Smith JR (1977) Lectin binding sites on the plasma membranes of rabbit spermatozoa. J Cell Biol 74:950–962
Perotti ME, Riva A (1988a) Sperm surface carbohydrates in Drosophila melanogaster. In: Chapman GP, Ainsworth CC, Chatham CJ (eds) Eukaryote cell recognition: concepts and model systems. Cambridge University Press, Cambridge, pp 153–162
Perotti ME, Riva A (1988b) Concanavalin A binding sites on the surface of Drosophila melanogaster sperm: a fluorescence and ultrastructural study. J Ultrastruct Res 100:173–182
Rosati F (1985) Sperm-egg interactions in ascidians. In: Metz CB, Monroy A (eds) Biology of fertilization, vol 2. Academic Press, New York, pp 361–388
Rosati F, De Santis R, Monroy A (1978) Studies on fertilization II lectin binding to the gametes of Ciona intestinalis. Exp Cell Res 116:419–427
Ruiz-Bravo N, Lennarz WJ (1989) Receptors and membrane interactions during fertilization. In: Schatten H, Schatten G (eds) The molecular biology of fertilization. Academic Press, San Diego, pp 21–36
Sander K (1985) Fertilization and egg cell activation in insects. In: Metz CB, Monroy A (eds) Biology of fertilization, vol 2. Academic Press, New York, pp 409–430
Schwarz MA, Koehler JK (1979) Alterations in lectin binding to guinea pig spermatozoa accompanying in vitro capacitation and the acrosome reaction. Biol Reprod 21:1295–1307
Shalgi R, Matityahu A, Nebel L (1986) The role of carbohydrates in sperm-egg interaction in rats. Biol Reprod 34:446–452
Szollozi A (1975) Electron microscope study of spermiogenesis in Locusta migratoria (Insecta. Orthoptera). J Ultrastruct Res 50:322–346
Wassarman PM (1988) Zona pellucida glycoproteins. Annu Rev Biochem 57:415–442
Yanagimachi R (1988) Mammalian fertilization. In: Knobil E, Neill JD (eds) The physiology of reproduction, vol I. Raven Press, New York, pp 135–185
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Esponda, P., Guerra, R. Plasma-membrane glycoproteins during spermiogenesis and in the spermatozoa of some Orthoptera. Cell Tissue Res 264, 507–513 (1991). https://doi.org/10.1007/BF00319040
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319040