Summary
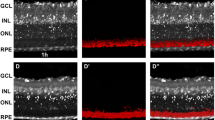
Stress proteins are thought to play an important role in cellular development and in survival mechanisms. We compared the immunolocalization of the 70-kDa stress protein (SP70) in the ocular tissue of the normal Sprague-Dawley (SD) rat with that in the Royal College of Surgeons (RCS) rat with retinal dystrophy. SP70 was present in the maturing ocular tissues of both rat strains. However, once retinal degeneration began in the RCS rat, the retinal pigment epithelium and photoreceptor cells showed increased immunostaining for SP70 over that observed in age-matched SD rats. In late stages of retinal degeneration, immunostaining for SP70 was considerably reduced in the RCS retina, whereas normal distribution of immunostaining for SP70 in the SD retina was preserved, albeit decreased, through postnatal day 180. The optic nerve, ciliary body, and corneal epithelium were also influenced by the dystrophic disease condition, although the pattern of changes in SP70 immunostaining differed for each tissue. These results suggest that the genetic defect in the RCS rat produces a state of metabolic stress in all ocular tissues as the degeneration progresses, but that the subsequent rise in ocular SP70 is insufficient to prevent progression of the disease.
Similar content being viewed by others
References
Barbe MF, Tytell M, Gower DJ, Welch WJ (1988) Hyperthermia protects against light damage in the rat retina. Science 241:1818–1820
Beckmann RP, Mizzen LA, Welch WJ (1990) Interaction of Hsp70 with newly synthesized proteins: implications for protein folding and assembly. Science 248:850–854
Bok D, Hall MO (1971) The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol 49:664–682
Bourne MC, Campbell DA, Tansley K (1938) Hereditary degeneration of the rat retina. Br J Ophthalmol 22:613–623
Brown IR, Lowe DG, Moran LA (1985) Expression of heat shock genes in fetal and maternal rabbit brain. Neurochem Res 10:1277–1284
Chapell TG, Welch WJ, Schlossman DM, Palter KB, Schlesinger MJ, Rothman JE (1986) Uncoating ATPase is a member of 70 kilodalton family of stress proteins. Cell 45:3–13
Chirico WJ, Waters MG, Blobel G (1988) 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 332:805–810
Clark BD, Brown IR (1985) Axonal transport of a heat shock protein in the rabbit visual system. Proc Natl Acad Sci USA 82:1281–1285
Cook-Moody J, Gaur V, Sheedlo H, Tytell M, Turner JE (1990) Heat shock protein expression in RCS rats: Multiple hyperthermic exposures do not protect photoreceptor cells from degeneration (abstract). Invest Ophthalmol Vis Sci [Suppl] 31:595
Craig EA (1985) The heat shock response. CRC Crit Rev Biochem 18:239–280
Dash A, Zelenka PS (1990) Expression of SP70 mRNA in the embryonic chicken lens (abstract). Invest Ophthalmol Vis Sci [Suppl] 31:507
Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R (1988) A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332:800–805
Dowling JE, Sidman RL (1962) Inherited retinal dystrophy in the rat. J Cell Biol 14:73–109
Essner E, Gorrin G (1979) An electron microscopic study of macrophages in rats with inherited retinal dystrophy. Invest Ophthalmol Vis Sci 18:11–25
Hartl FU, Neupert W (1990) Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science 247:930–938
LaVail MM, Sidman RL, O'Neil D (1972) Photoreceptor-pigment epithelial cell relationships in rats with inherited retinal degeneration. Radioautographic and electron microscope evidence for a dual source of extra lamellar material. J Cell Biol 53:185–209
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–6677
Milarski KL, Morimoto RI (1986) Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci USA 83:9517–9521
Pelham HRB (1986) Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 46:959–961
Pelham HRB (1988) Heat-shock proteins: coming in from the cold. Nature 332:776–777
Pinhasi-Kimhi O, Michalovitz D, Ben-Zeev A, Oren M (1986) Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature 320:182–185
Riabowol KT, Mizzen LA, Welch WJ (1988) Heat shock is lethal to fibroblasts microinjected with antibodies against hsp 70. Science 242:433–436
Schlesinger MJ (1986) Heat shock proteins: the search for functions. J Cell Biol 103:321–325
Schlesinger MJ, Ashburner M, Tissieres A (eds) (1982) Heat shock: from bacteria to man. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 1–440
Subjeck JR, Shyy TT (1986) Stress protein systems of mammalian cells. Am J Physiol 250 (Cell Physiol 19):C1-C17
Tamai M, O'Brien PJ (1979) Retinal dystrophy in the RCS rat: in vivo and vitro studies of phagocytic action of the pigment epithelium on the shed rod outer segments. Exp Eye Res 28:399–411
Tomasovic SP (1989) Functional aspect of the mammalian heatstress protein response. Life Chem Rep 7:33–63
Tytell M, Barbe MF (1987) Synthesis and axonal transport of heat shock proteins. In: Smith RS, Bisby MA (eds) Neurology and neurobiology, vol 25. Axonal transport. Liss, New York, pp 473–492
Ungewickell E (1985) The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J 4:3385–3391
Waegh S de, Brady ST (1989) Axonal transport of a clathrin uncoating ATPase (HSC70): a role for HSC70 in the modulation of coated vesicle assembly in vivo. J Neurosci Res 23:433–440
Yamaguchi K, Yamaguchi K, Turner JE (1990a) Ciliary body degeneration in the Royal College of Surgeons rat (abstract). Invest Ophthalmol Vis Sci [Suppl] 31:446
Yamaguchi K, Yamaguchi K, Turner JE (1990b) Corneal and vitreal abnormalities accompanying retinal dystrophy in the Royal College of Surgeons rat (abstract). Invest Ophthalmol Vis Sci [Suppl] 31:446
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamaguchi, K., Gaur, V.P., Tytell, M. et al. Ocular distribution of 70-kDa heat-shock protein in rats with normal and dystrophic retinas. Cell Tissue Res 264, 497–506 (1991). https://doi.org/10.1007/BF00319039
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319039