Summary
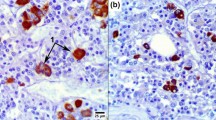
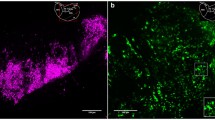
Semi-thin sections of three-dimensional reaggregates from adult female rat pituitary, cultured in serum-free defined medium, were stained for prolactin, gonadotropin, thyrotropin, growth hormone and S-100, using the double immunolabelling technique. The frequency of juxtaposition between lactotrophs and gonadotrophs was enumerated and compared with the expected frequency at random distribution of polygonal cell profiles in a hexagonal configuration. The proportions of lactotrophs and gonadotrophs in the aggregate sections were determined using stereometrical analysis. The observed frequency of juxtaposition did not differ significantly from the expected frequency. Hence, no reason was found to assume a selective adhesion between lactotrophs and gonadotrophs in adult female rat pituitary reaggregates. A constant proportion of lactotrophs was found to meet the criteria of a cup-shaped morphology, and 70%±9% (mean ±S.D.) of these so-called cupshaped lactotrophs were found to be juxtaposed at their concave side to gonadotrophs. Administration of 0.01 nM 17β-oestradiol to the culture medium resulted in a significant reduction of the proportion of cup-shaped lactotrophs but did not affect the selectivity of juxtaposition to gonadotrophs. The selectivity of juxtaposition between cup-shaped lactotrophs and gonadotrophs may be the morphological correlate of the functional relationship between these cells, which are known to be involved in an intra-pituitary paracrine communication system.
Similar content being viewed by others
References
Allaerts W, Denef C (1989) Regulatory activity and topological distribution of folliculo-stellate cells in rat anterior pituitary cell aggregates. Neuroendocrinology 49:409–418
Allaerts W, Carmeliet P, Denef C (1990) New perspectives in the function of pituitary folliculo-stellate cells. Mol Cell Endocrinol 71:73–81
Baes M, Denef C (1987) Evidence that stimulation of growth hormone release by epinephrine and vascoactive intestinal peptide is based on cell-to-cell communication in the pituitary. Endocrinology 120:280–290
Baes M, Allaerts W, Denef C (1987) Evidence for functional communication between folliculo-stellate cells and hormone-secreting cells in perifused anterior pituitary cell aggregates. Endocrinology 120:685–691
Bégeot M, Hemming FJ, Dubois PM, Combarnous Y, Dubois MP, Aubert ML (1984) Induction of pituitary lactotrope differentiation by luteinizing hormone α subunit. Science 226:566–568
Bodine SC, Garfinkel A, Roy RR, Edgerton VR (1988) Spatial distribution of motor unit fibers in the cat soleus and tibialis anterior muscles: local interactions. J Neurosci 8:2142–2152
Cocchia D, Miani N (1980) Immunocytochemical localization of the brain-specific S-100 protein in the pituitary gland of adult rat. J Neurocytol 9:771–782
Denef C (1986) Paracrine interactions in the anterior pituitary. In: Franchimont P (ed) Paracrine control. Clinics in endocrinology and metabolism, vol 15. Saunders, London, pp 1–32
Denef C, Andries M (1983) Evidence for paracrine interaction between gonadotrophs and lactotrophs in pituitary cell aggregates. Endocrinology 112:813–822
Denef C, Schramme C (1985) Regulation of prolactin release by angiotensin. Horm Res 22:135–141
Denef C, Hautekeete E, Dewolf A, Van Der Schueren B (1978) Pituitary basophils from immature male and female rats: distribution of gonadotrophs and thyrotrophs as studied by unit gravity sedimentation. Endocrinology 103:724–735
Denef C, Hautekeete E, Dewals R, Dewolf A (1980) Differential control of luteinizing hormone and follicle-stimulating hormone secretion by androgens in rat pituitary cells in culture: functional diversity of subpopulations separated by unit gravity sedimentation. Endocrinology 106:724–729
Denef C, Baes M, Schramme C (1986) Paracrine interactions in the anterior pituitary: role in the regulation of prolactin and growth hormone secretion. In: Ganong WF, Martini L (eds) Frontiers in neuroendocrinology, vol 9. Raven Press, New York, pp 115–148
Horvath E, Kovacs K, Ezrin C (1977) Junctional contact between lactotrophs and gonadotrophs in the rat pituitary. IRCS Medical Sci 5:511
Hsu S-M, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Ishikawa H (1969) Isolation of different type of anterior pituitary cells in rats. Endocrinol Jpn 16:517–529
Jones TH, Brown BL, Dobson PRM (1988) Evidence that angiotensin II is a paracrine agent mediating gonadotrophin-releasing hormone-stimulated inositol phosphate production and prolactin secretion in the rat. J Endocrinol 116:367–371
Mason DY, Abdulaziz Z, Falini B, Stein H (1983) Double immunoenzymatic labeling. In: Polak JM, Van Noorden S (eds) Immunocytochemistry, practical applications in pathology and biology. Wright, Bistrol, England, pp 113–128
Morris CS, Hitchcock E (1985) Immunocytochemistry of folliculostellate cells of normal and neoplastic human pituitary gland. J Clin Pathol 38:481–488
Nakajima T, Yamaguchi H, Takahashi K (1980) S 100 protein in folliculo-stellate cells of the rat pituitary anterior lobe. Brain Res 191:523–531
Nakane PH (1970) Classifications of anterior pituitary cell types with immunoenzyme histochemistry. J Histochem Cytochem 18:9–20
Nakane PH (1975) Identification of anterior pituitary cells by immunoelectron microscopy. In: Tixier-Vidal A, Farquhar MG (eds) The anterior pituitary. Academic Press, New York, pp 45–61
Neill JD (1980) Neuroendocrine regulation of prolactin secretion. In: Martini L, Ganong WF (eds) Frontiers in neuroendocrinology, vol 6. Raven Press, New York, pp 129–155
Nogami H, Yoshimura F (1982) Fine structural criteria of prolactin cells identified immunohistochemically in the male rat. Anat Rec 202:261–274
Padmanabhan V, Leung K, Convey EM (1982) Ovarian steroids modulate the self-priming effect of luteinizing hormone-releasing hormone on bovine pituitary cells in vitro. Endocrinology 110:717–721
Sato S (1980) Postnatal development, sexual difference and sexual cycle variation of prolactin cells in rats: special reference to the topographic affinity to a gonadotroph. Endocrinol Jpn 27:573–583
Siegel S (1956) Nonparametric statistic for the behavioral sciences. International Student Edition, McGraw-Hill, New York
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, San Francisco
Steinberg MS (1970) Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool 173:395–434
Thompson, D'arcy W (abridged edition by Bonner JT, 1961) On growth and form. Cambridge University Press, Cambridge
Van der Schueren B, Denef C, Cassiman J-J (1982) Ultrastructural and functional characteristics of rat pituitary cell aggregates. Endocrinology 110:513–523
Vandesande F (1983) Immunohistochemical double staining techniques. In: Cuello AC (ed) Immunohistochemistry. IBRO, Wiley, Chichester New York, pp 257–272
Weibel ER (1979) Stereological Methods. 1. Practical methods for biological morphometry. Academic Press, New York
Wilfinger WW, Larsen WJ, Downs TR, Wilbur DL (1984) An in vitro model for studies of intercellular communication in cultured rat anterior pituitary cells. Tissue Cell 16:483–497
Zamboni L, De Martino C (1967) Buffered picric acid-formaldehyde: a new, rapid fixative for electron microscopy. J Cell Biol 35:148A
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Allaerts, W., Mignon, A. & Denef, C. Selectivity of juxtaposition between cup-shaped lactotrophs and gonadotrophs from rat anterior pituitary in culture. Cell Tissue Res 263, 217–225 (1991). https://doi.org/10.1007/BF00318763
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318763