Abstract
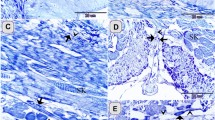
In the present study, we characterized intra-epithelial leukocytes in the digestive tract of chickens during postnatal development. Their phenotype was characterized by monoclonal antibodies in cryostat sections and the numbers of the different cell-types were counted in the epithelium of the esophagus, proventriculus, duodenum, jejunum, cecum, and colon. All intra-epithelial leukocytes bore the leukocyte-common antigen CD45; 35% were T lymphocytes, and 50% bore a B-cell marker. However, no immunoglobulin-bearing cells were detected in the epithelium. Monocytes and macrophages were found only in the epithelium of the esophagus. A remaining population of non-B, non-T, non-monocyte cells (15%) was present in all parts of the digestive tract. The number of intra-epithelial leukocytes was greatest in the duodenum and jejunum, and decreased in the proximal part of the cecum and in the colon. Intra-epithelial leukocytes were only sporadically detected in the proventriculus. The total number of intra-epithelial leukocytes increased until 8 weeks after hatching and then decreased at 18 months. In the esophagus, the total number of intra-epithelial leukocytes changed little during aging. We found that the intra-epithelial leukocytes of chickens and rodents are distinct in that chicken intra-epithelial leukocytes comprise a cell population that bears a B-cell antigen but that lacks surface immunoglobulins.
Similar content being viewed by others
References
Abo T, Balch CM (1981) A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol 127:1024–1029
Arnaud-Battandier F, Lawrence EC, Blaese RM (1980) Lymphoid populations of gut mucosa in chickens. Dig Dis Sci 25:252–259
Bäck O (1972) Studies on the lymphocytes in the intestinal epithelium of the chickens. 1. Ontogeny. Acta Pathol Microbiol Scand [A] 80:84–90
Bucy RP, Chen CH, Cihak J, Losch U, Cooper MD (1988) Avian T cells expressing γδ receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol 141:2200–2205
Bucy RP, Chen CH, Cooper MD (1990) Development of cytoplasmic CD3+/T cell receptor-negative cells in the peripheral lymphoid tissues of chickens. Eur J Immunol 20:1345–1350
Burck HC (1973) Histologische Technik, 3rd edn. Thieme, Stuttgart
Buret A, Gall DG, Nation PN, Olson ME (1990) Intestinal protozoa and epithelial cell kinetics, structure and function. Immunol Today 6:375–380
Cerf-Bensussan N, Guy-Grand D, Griscelli C (1985) Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut 26:81–88
Ernst PB, Befus AD, Bienenstock J (1985) Leucocytes in the intestinal epithelium: an unusual immunological compartment. Immunol Today 6:50–55
Ferguson A (1977) Intraepithelial lymphocytes of the small intestine. Gut 18:921–937
Ferguson A, Parrott DMV (1972) The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes of the mouse. Clin Exp Immunol 12:477–488
Gibson PR, Hermanowics A, Verhaar HJJ, Ferguson DJP, Lopez Bernal A, Jewell DP (1985) Isolation of intestinal mononuclear cells: factors which affect lymphocyte viability and function. Gut 26:60–68
Hanglow AC, Ernst PB, Bienenstock J (1990) Intraepithelial leucocytes, murine. In: Jones TC, Ward JM, Mohr U, Hunt RD (eds) Hemopoietic system. Springer, Berlin Heidelberg New York, pp 315–322
Jeurissen SHM, Janse EM, Ekino S, Nieuwenhuis P, Koch G, Boer GF de (1988a) Monoclonal antibodies as probes for defining cellular subsets in the bone marrow, thymus, bursa of Fabricius, and spleen of the chicken. Vet Immunol Immunopathol 19:225–238
Jeurissen SHM, Janse EM, Koch G, Boer GF de (1988b) The monoclonal antibody CVI-ChN1-68.1 recognizes cells of the monocyte-macrophage lineage in chickens. Dev Comp Immunol 12:855–864
Jeurissen SHM, Janse EM, Koch G, Boer GF de (1989) Postnatal development of mucosa-associated lymphoid tissues in chickens. Cell Tissue Res 258:119–124
Kitagawa H, Hashimoto Y, Kon Y, Kudo N (1988) Light and electron microscopic studies on chicken intestinal globule leukocytes. Jpn J Vet Res 36:83–117
Lawrence EC, Arnaud-Battandier F, Koski IR, Dooley NJ, Muchmore AV, Blaese MR (1979) Tissue distribution of immunoglobulin-secreting cells in normal and IgA-deficient chickens. J Immunol 123:1767–1771
Lillehoj HS, Bacon LD (1991) Increase of intestinal intraepithelial lymphocytes expressing CD8 antigen following challenge infection with Eimeria acervulina. Avian Dis 35:294–301
Lillehoj HS, Chung KS (1992) Postnatal development of T-lymphocyte subpopulations in the intestinal intraepithelium and lamina propria in chickens. Vet Immunol Immunopathol 31:347–360
Lillehoj HS, Lillehoj EP, Weinstock D, Schat KA (1988) Functional and biochemical characterization of avian T lymphocyte antigen identified by monoclonal antibodies. Eur J Immunol 18:2059–2065
Lyscom N, Brueton MJ (1982) Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology 45:775–783
Mead R, Curnow RN (1983) Statistical methods in agriculture and experimental biology. Chapman and Hall, London
Mowat A McI (1990) Human intraepithelial lymphocytes. Springer Semin Immunopathol 12:165–190
Nagi AM, Babiuk LA (1989) Characterization of surface markers of bovine gut mucosal leuocytes using monoclonal antibodies. Vet Immunol Immunopathol 22:1–14
Noteborn MHM, Boer GF de, Roozelaar DJ van, Karreman C, Kranenburg O, Vos JG, Jeurissen SHM, Hoeben RC, Zantema A, Koch G, Ormondt H van, Eb AJ van der (1991) Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J Virol 65:3131–3139
Paramithiotis E, Tkalec L, Ratcliffe MJH (1991) High levels of CD45 are coordinately expressed with CD4 and CD8 on avian thymocytes. J Immunol 147:3710–3717
Petit A, Ernst PB, Befus AD, Clark DA, Rosenthal KL, Ishizaka T, Bienenstock J (1985) Murine intestinal intraepithelial lymphocytes I. Relationship of a novel Thy-1-,Lyt-1-, Lyt-2+, granulated subpopulation to natural killer cells and mast cells. Eur J Immunol 15:211–215
Pink JR, Rijnbeek AM (1983) Monoclonal antibodies against chicken lymphocyte surface antigens. Hybridoma 2:287–296
Selby WS, Janossy G, Jewell DP (1981) Immunohistological characterization of intraepithelial lymphocytes of the human gastrointestinal tract. Gut 22:169–176
Selby WS, Janossy G, Bofill M, Jewell DP (1984) Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut 25:32–40
Van der Heijden FL (1986) Mucosal lymphocytes in the rat small intestine: phenotypical characterization in situ. Immunology 59:397–399
Veromaa T, Vainio O, Eerola E, Toivanen P (1988) Monoclonal antibodies against chicken Bu-1a and Bu-1b alloantigens. Hybridoma 7:41–48
Welsh RM, Haspel MV, Parker DC, Holmes KV (1986) Natural cytotoxicity against mouse hepatitis virus-infected cells. II. A cytotoxic effector cell with a B lymphocyte phenotype. J Immunol 136:1454–1460
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vervelde, L., Jeurissen, S.H.M. Postnatal development of intra-epithelial leukocytes in the chicken digestive tract: phenotypical characterization in situ. Cell Tissue Res 274, 295–301 (1993). https://doi.org/10.1007/BF00318748
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318748