Summary
In teleost fish, the visceral sensory nuclei of the medulla are clearly separated into gustatory lobes and a general visceral sensory nucleus. Those branches of the vagus nerve which innervate the orobranchial cavity terminate in the vagal gustatory lobe, while the general visceral component of the vagus nerve terminates in the separate general visceral nucleus. In goldfish, the vagal lobe is a complex, laminated structure containing both motor and sensory elements.
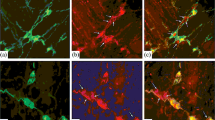
Transection of the vagus nerve results in distinct changes in the pattern of acetylcholinesterase staining and substance-P-like (SPL) immunoreactivity in the vagal lobe of goldfish. Following vagotomy, cholinesterase activity is eliminated from layers 4 and 6, both being layers in which primary gustatory afferent fibers terminate. In addition, SPL immunoreactive fibers disappear from the capsular root of the vagus nerve. These results indicate that the primary afferent input to the gustatory lobe involves at least two cytochemically distinct fiber types, one containing substance-P-immunoreactive material and the other containing or inducing acetylcholinesterase activity.
Vagotomy also affects immunostaining and cholinesterase activity of the motonuerons deep in the vagal lobe. Following nerve transection, acetylcholinesterase activity is diminished, and SPL-immunoreactivity increased in the affected motoneurons. Similar changes were observed in axotomized motoneurons of other cranial nerve nuclei.
Similar content being viewed by others
References
Berod A, Hartman BK, Pujol JF (1981) Importance of fixation in immunohistochemistry: Use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem 29:844–850
Chubb IW, Hodgson AJ, White GH (1980) Acetylcholinesterase hydrolyzes substance P. Neuroscience 5:2065–2072
Contestabile A (1975) Histochemical study on the distribution of some enzyme activities in the vagal and facial lobes of the goldfish, Carassius auratus. Histochemistry 44:123–132
Cuello AC, Galfre G, Milstein C (1979) Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 76:3532–3536
Eskay RL, Furness JF, Long RT (1981) Substance P activity in the bullfrog retina: localization and identification in several vertebrate species. Science 212:1049–1051
Finger TE (1978) Gustatory pathways in the bullhead catfish: II. Facial lobe connections. J Comp Neurol 180:691–706
Finger TE (1981) Enkephalin-like immunoreactivity in the gustatory lobes and visceral nuclei in the brains of goldfish and catfish. Neuroscience 6:2747–2758
Finger TE (1983) The gastatory system in teleost fish. In: Northcutt RG, Davis RE (eds) Fish neurobiology Vol I. Univ of Mich Press, Ann Arbor MI, pp 285–310
Friede RL (1966) Topographic brain chemistry. Academic Press, New York-London
Gamse R, Lembeck F, Cuello AC (1979) Substance P in the vagus nerve. Naunyn Schmiedebergs Arch Pharmacol 306:37–44
Geneser-Jensen FA, Blackstad, TW (1971) Distribution of acetylcholinesterase in the hippocampal region of the guinea pig. Z Zellforsch 114:460–481
Gillis RA, Helke CJ, Hamilton BL (1980) Evidence that substance P is a neurotransmitter of baro- and chemoreceptor afferents in nucleus tractus solitarius. Brain Res 181:476–481
Helke CJ, O'Donohue TL, Jacobowitz DM (1980) Substance P as a baro- and chemoreceptor afferent neurotransmitter: immunocytochemical and neurochemical evidence in the rat. Peptides 1:1–9
Herrick CJ (1905) Central gustatory paths in brains of bony fishes. J Comp Neurol 15:375–456
Kalia M, Mesulam MM (1980a) Brain stem projections of the sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol 193:435–466
Kalia M, Mesulam MM (1980b) Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac and gastrointestinal branches. J Comp Neurol 193:467–508
Katz DM, Karten HJ (1979) Substance P like immunoreactivity (SPLI) and target organ representation within the nucleus solitarius (nS) and vagal motor nucleus (DMN X). Abst. Soc Neurosci 5:530
Katz DM, Karten HJ (1983) Visceral representation within the nucleus of the tractus solitarius in the pigeon Columba livia. J Comp Neurol 218:42–73
Levey AI, Wainer BH, Mufson EJ, Mesulam M-M (1983) Colocalization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience 9:9–22
Lundberg JM, Hökfelt T, Nilsson G, Terenius L, Rehfeld J, Elde R, Said S (1978) Peptide neurons in the vagus, splanchnic and sciatic nerves. Acta Phy Scand 104:499–501
Lundberg JM, Hökfelt T, Anggard A, Pernow B, Emson P (1979) Immunohistochemical evidence for substance P immunoreactive nerve fibers in the taste buds of the cat. Acta Phys Scand 107:389–391
Maley B, Elde R (1982) Immunohistochemical localization of putative neurotransmitters within the feline nucleus tractus solitarii. Neuroscience 7:2469–2490
McLean IW, Nakane PK (1974) Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem 22:1077
Morita Y, Finger TE (1984) Differential laminar projection of palatal and gill arch taste buds in the goldfish. Abst Assoc Chemorecept Sci 6:99
Morita Y, Ito H, Masai H (1980) Central gustatory paths in the crucian carp, Carassius carassius. J Comp Neurol 191:119–132
Morita Y, Murakami T, Ito H (1983) Cytoarchitecture and topographic projections of the gustatory centers in a teleost Carassius carassius. J Comp Neurol 218:378–394
Nagy JI, Goedert M, Hunt SP, Bard A (1982) The nature of the substance P-containing nerve fibers in taste papillae of the rat tongue. Neuroscience 7:3137–3151
Navaratnam V, Lewis PR, Shute CCD (1968) Effects of vagotomy on the cholinesterase content of the preganglionic innervation of the rat heart. J Anat 103:225–232
Thoenen H (1974) Trans-synaptic enzyme induction. Life Sci 14:223–225
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Finger, T.E. Vagotomy induced changes in acetyl cholinesterase staining and substance P-like immunoreactivity in the gustatory lobes of goldfish. Anat Embryol 170, 257–264 (1984). https://doi.org/10.1007/BF00318729
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318729