Summary
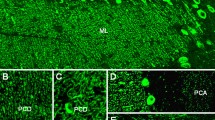
The secretory pathway of the complex-type glycoprotein specific to the subcommissural organ (SCO) was examined using the monoclonal antibody (Mab) C1B8A8. Immunoreactive material was revealed in various compartments of the secretory ependymocyte, i.e., the endoplasmic reticulum, the Golgi area and the secretory vacuoles. In addition, immunoreactive material was also observed in the ventricular cavity. Evidence of a release both at the apical lining and at the basal process of the SCO ependymocytes suggests that the same protein could be secreted into the cerebrospinal fluid and the perivascular spaces. After immunoaffinity chromatography of soluble extracts of the SCO on Mab C1B8A8 immunoadsorbent columns, three glycopeptides were identified on Western blots; they were concanavalin A (Con A)-positive (88, 54 and 34 kDa) and wheat-germ agglutinin (WGA)-positive (54 and 34 kDa). The Con A-positive glycopeptide (88 kDa) is probably related to the high-mannose-type glycoprotein, the precursor form of the secreted compound, whereas the 54 kDa-glycopeptide that is both Con A- and WGA-positive could represent an intermediate form. The 34 kDa-glycopeptide that is strongly WGA-positive could be related to the monomeric form of the secreted compound. These three glycopeptides were not revealed in eluted fractions of soluble extracts of the ependyma that served as control.
Similar content being viewed by others
References
Batteiger B, Newhall WJ, Jones RB (1982) The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Method 55:297–307
Bruel MT, Meiniel R, Meiniel A, David D (1987) Ontogenetical study of the chick embryo subcommissural organ by lectin histofluorescence and electron microscopy. J Neural Transm 70:145–168
Duchier-Liris N (1991) Différenciation cellulaire d'une structure épendymaire, l'organe sous-commissural; préparation d'anticorps monoclonaux et analyse immunologique et biochimique des sécrétions glycoprotéiques chez les Mammifères. Thesis, Faculté de Médecine, Université d'Auvergne, Clermont-Ferrand, France
Glass WF, Briggs RC, Hnilica LS (1981) Use of lectins for detection of electrophoretically separated glycoproteins transferred on nitrocellulose sheets. Anal Biochem 115:219–224
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–302
Hauser R (1972) Morphogenetic action of the subcommissural organ on tail regeneration in Xenopus larvae. Wilhelm Roux Archiv 169:170–184
Hess J, Sterba G (1973) Studies concerning the function of the complex subcommissural organ-liquor fibre: the binding ability of the liquor fibre to pyrocatechin derivatives and its functional aspects. Brain Res 58:303–312
Hofer HO (1987) The terminal organ of the subcommissural complex of the chordates: definition and perspectives. Gegenbaurs Morphol Jahrb Leipzig 133:217–226
Karoumi A (1990) Mise en évidence et caractérisation par des méthodes immunologiques de sécrétions glycoprotéiques au niveau du diencéphale dorsal. (Etude particulière d'une structure circumventriculaire: l'organe sous-commissural du bœuf adulte et de l'embryon de poulet). Thesis, Faculté des Sciences, Université Blaise Pascal, Clermont-Ferrand, France, no UD 199
Karoumi A, Meiniel R, Croisille Y, Belin MF, Meiniel A (1990a) Glycoptrotein synthesis in the subcommissural organ of the chick embryo. I. An ontogenetical study using specific antibodies. J Neural Transm 79:141–153
Karoumi A, Croisille Y, Croisille F, Meiniel R, Belin MF, Meiniel A (1990b) Glycoprotein synthesis in the subcommissural organ of the chick embryo. II. An immunochemical study. J Neural Transm 80:203–212
Karoumi A, Meiniel R, Belin MF, Meiniel A (1991) A comparative immunocytochemical and immunochemical analysis of glycoproteins synthesized in the bovine subcommissural organ. J Neural Transm (in press)
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lis H, Sharon N (1986) Lectins as molecules and as tools. Annu Rev Biochem 55:35–67
Lösecke W, Naumann W, Sterba G (1984) Preparation and discharge of secretion in the subcommissural organ of the rat. An electron-microscopic immunocytochemical study. Cell Tissue Res 235:201–206
Lösecke W, Naumann W, Sterba G (1986) Immuno-electron-microscopic analysis of the basal route of secretion in the subcommissural organ of the rabbit. Cell Tissue Res 224:449–456
Meiniel R, Meiniel A (1985) Analysis of the secretions of the subcommissural organs of several vertebrate species by use of fluorescent lectins. Cell Tissue Res 239:359–364
Meiniel R, Molat JL, meiniel A (1986) Concanavalin A-binding glycoproteins in the subcommissural and the pineal organ of the sheep (Ovis aries). A fluorescence-microscopic and electrophoretic study. Cell Tissue Res 245:605–613
Meiniel A, Molat JL, Meiniel R (1988a) Complex-type glycoproteins synthesized in the subcommissural organ of mammals. Light- and electron-microscopic investigations by use of lectins. Cell Tissue Res 253:383–395
Meiniel R, Duchier N, Meiniel A (1988b) Monoclonal antibody C1B8A8 recognizes a ventricular secretory product elaborated in the bovine subcommissural organ. Cell Tissue Res 254:611–615
Meiniel R, Molat JL, Duchier-Liris N, Meiniel A (1990) Ontogenesis of secretory epithelium of the bovine subcommissural organ. A histofluorescence study using lectins and monoclonal antibodies. Dev Brain Res 55:171–180
Oksche A (1969) The subcommissural organ. Acta Zool (Stockh) 39:71–102
Rodríguez EM, Oksche A, Hein S, Rodriguez S, Yulis R (1984a) Comparative immunocytochemical study of the subcommissural organ. Cell Tissue Res 237:427–441
Rodríguez EM, Oksche A, Hein S, Rodríguez S, Yulis R (1984b) Spatial and structural interrelationships between secretory cells of the subcommissural organ and blood vessels. An immunocytochemical study. Cell Tissue Res 237:443–449
Rodríguez EM, Herrera H, Peruzzo B, Rodríguez S, Hein S, Oksche A (1986) Light- and electron-microscopic lectin histochemistry and immunocytochemistry of the subcommissural organ: evidence for processing of the secretory material. Cell Tissue Res 243:545–559
Rodríguez EM, Hein S, Rodríguez S, Herrera H, Peruzzo B, Nualart F, Oksche A (1987) Analysis of the secretory products of the subcommissural organ. In: Scharrer B, Korf H-W, Hartwig H-G (eds) Functional morphology of neuroendocrine systems. Springer, Berlin Heidelberg New York, pp 189–202
Rühle HJ (1971) Anomalien im Wachstum der Achsenorgane nach experimenteller Ausschaltung des Komplexes Subcommissuralorgan-Reissnerscher Faden. Untersuchungen am Rippenmolch (Pleurodeles waltlii Michah 1830). Acta Zool (Stockh) 52:23–68
Schoebitz K, Garrido O, Heinrichs MS, Peer L, Rodríguez EM (1986) Ontogenetical development of the chick and duck subcommissural organ. An immunocytochemical study. Histochemistry 81:31–40
Sterba G, Kleim I, Naumann W, Petter H (1981) Immunocytochemical investigation of the subcommissural organ in the rat. Cell Tissue Res 218:659–662
Sterba G, Kiessig C, Naumann W, Petter H (1982) The secretion of the subcommissural organ. A comparative immunocytochemical investigation. Cell Tissue Res 226:427–439
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications. Procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meiniel, R., Duchier-Liris, N., Molat, J.L. et al. The complex-type glycoprotein secreted by the bovine subcommissural organ: an immunological study using C1B8A8 monoclonal antibody. Cell Tissue Res 266, 483–490 (1991). https://doi.org/10.1007/BF00318589
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318589