Abstract
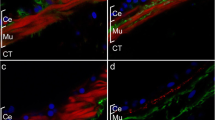
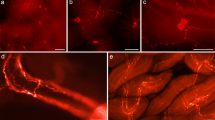
During regeneration of the neural ganglion in Ciona intestinalis, the pattern of reappearance of some peptidergic cells is similar to the ontogenetic patterns exhibited by these cell types during normal post-metamorphic development. Using a specific antiserum to gamma-aminobutyric acid (GABA), we describe here the appearance of GABA-ergic cells in Ciona during both post-metamorphic development and regeneration of the neural ganglion following total ablation. Post-metamorphic animals were divided into the categories: 1, 3–5, 6–10, 11–15 and 23–27 mm in body length. Regeneration was monitored at 12, 15, 18, 21, 28 and 56 days post ablation. The first appearance of GABA-like immunoreactive cells during normal development were at the 3 to 5-mm stage where they were seen as discrete cells, without processes, evenly distributed in the cortical region throughout the ganglion. Fibres were first seen at the 6 to 10-mm stage. As development proceeded, GABA-like immunoreactive cells became more concentrated near the nerve root exits and along the dorsal rind of the ganglion. In regenerating ganglia, GABA was first detected at 18–21 days post ablation, in cells that lacked any obvious processes and that were distributed in all regions of the ganglion. At 28 days post ablation, processes could be detected in the neuropile, and after 56 days GABA cells were found predominantly in the same regions as in the normally developing adult ganglion. Although the overall pattern reflects that in a normal adult, a few differences were detectable. For example, rather more GABAergic cells were concentrated ventrally in the ganglion close to the neural gland.
Similar content being viewed by others
References
Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, Mckerracher L, Villegasperez MP, Vidalsanz M, Carter DA (1991) Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Phil Trans Roy Soc [B] 331:337–343
Barnard EA, Darlison MG, Seeburg P (1987) Molecular biology of the GABAA receptor: the receptor/channel superfamily. Trends Neurosci 10:502–509
Berrill NJ (1995) The origin of vertebrates. Oxford University Press, London
Blackshawe SE (1987) Organisation and development of the peripheral nervous system in annelids. In: Ali M (ed) Nervous systems in invertebrates. Plenum Press, New York London, pp 265–302
Bollner T, Storm-Mathisen J, Ottersen OP (1991) GABA-like immunoreactivity in the nervous system of Oikopleura dioica (Appendicularia). Biol Bull 180:119–124
Bollner T, Beesley PW, Thorndyke MC (1992) The pattern of substance P- and cholecystokinin-like immunoreactivity during regeneration of the neural complex in the ascidian Ciona intestinalis. J Comp Neurol 325:572–580
Bollner T, Beesley PW, Thorndyke MC (1993) Substance P- and cholecystokinin-like immunoreactivity during post-metamorphic development of the central nervous system in the ascidian Ciona intestinalis. Cell Tissue Res 272:545–552
Dybern BI (1965) The life cycle of Ciona intestinalis (L.) f. typica in relation to the environmental temperature. Oikos 16:109–131
Elphick MR, Moss C, Thorndyke MC (1990) The SALMF amide neuropeptides: in search of a biological activity. Regul Pept 30:41
Elwyn A (1937) Some stages in the development of the neural complex in Ecteinascida turbinata. Bull Neurol Inst NY 6:163–177
Ermak TH (1975) An autoradiographic demonstration of blood cell renewal in Styela clava (Urochordata: Ascidacea). Experientia 31:837–839
Florey E (1963) Acetylcholine and cholinesterase in tunicates. Comp Biochem Physiol 8:327–330
Florey E (1967) Cholinergic neurons in tunicates: an appraisal of the evidence. Comp Biochem Physiol 22:617–627
Florey E, Cahill MA, Rathmayer M (1975) Excitatory actions of GABA and acetylcholine in sea urchin tube feet. Comp Biochem Physiol [C] 51:5812
Fry KR, Chen NX, Glazebrook PA, Lam DMK (1991) Postnatal development of ganglion cells in the rabbit retina-characterizations with AB5 and GABA antibodies. Dev Brain Res 61:45–53
Georges D (1977) Analyse, fonctionelle du complex neurale chez Ciona intestinalis (Tunicer, Ascidiacé). Gen Comp Endocrinol 32:454–473
Georges D (1985) Presence of cells resembling serotonergic elements in four species of tunicates. Cell Tissue Res 242:341–348
Harvey RJ, Vreugdenhil E, Zaman SH, Bhandal NS, Usherwood PNR, Barnard EA, Darlison MG (1991) Sequence of a functional invertebrate GABAA receptor subunit which can form a chimeric receptor with a vertebrate a subunit. EMBO J 10:3239–3245
Hatt H, Franke C (1989) Wide range transmitter sensitivities of a crustacean chloride channel. In: Anderson PAV (ed) Evolution of the first nervous systems. Plenum Press, New York, pp 167–176
Hodgson AJ, Penke B, Erdei A, Chubb IA, Somogyi P (1985) Antisera to gamma-aminobutyric acid. 1. Production and characterization using a new model system. J Histochem Cytochem 33:229–239
Kawamura K, Nakauchi M (1991) Homeostatic integration of stem cell dynamics during palleal budding of ascidians. Zool Sci 8:11–22
Landis SC (1990) Target regulation of neurotransmitter phenotype. Trends Neurosci 13:344–350
Lender T, Bouchard-Madrelle C (1964) Êtudes expérimentale de la régénération du complex neural de Ciona intestinalis (Prochordé). Bull Soc Zool 89:546–554
Markman B (1958) On the peripheral nervous system of ascidians. Acta Zool 39:13–18
Meyer EP, Matute C, Streit P, Nässel DR (1986) Insect optic lobe neurons identifiable with monoclonal antibodies to GABA. Histochemistry 84:207–216
Millar RH (1953) Ciona (LMBC Memoirs No 53). University Press, Liverpool
Morse ANC, Morse DE (1984) GABA-mimetic molecules from Porphyra (Rhodophyta) induce metamorphosis of Haliotis (Gastropoda larvae). Hydrobiologia 116/117:155–158
Morse DE, Duncan H, Hooker N, Baloun A, Young G (1980) GABA induces behavioral and developmental metamorphosis in planctonic molluscan larvae. Fed Proc 39:3237–3241
Nicol D, Meinertzhagen IA (1991) Cell counts and maps in the larval central nervous system of the ascidian Ciona intestinalis (L.). J Comp Neurol 309:415–429
Olsen RW, Tobin AJ (1990) Molecular biology of the GABAA receptor. FASEB J 4:1469–1480
Osborne NN, Neuhoff V, Ewers E, Robertson HA (1979) Putative neurotransmitters in the cerebral ganglia of the tunicate Ciona intestinalis. Comp Biochem Physiol [C]. 63:209–213
Ottersen OP, Storm-Mathisen J, Strømhaug J (1986) Evaluation of the immunocytochemical method for amino acids. Med Biol 64:147–158
Purves D, Lichtman JW (1985) Principles of neural development. Sinauer, Sunderland, Mass
Raftos DA, Cooper EL (1991) Proliferation of lymphocyte-like cells from the solitary tunicate, Styela clava, in response to allogenic stimuli. J Exp Zool 260:391–400
Raftos DA, Cooper EL, Habicht GS, Beck G (1991) Invertebrate cytokines-tunicate cell proliferation stimulated by an interleukin-1-like molecule. Proc Natl Acad Sci USA 88:9518–9522
Roberts A, Dale N, Ottersen OP, Storm-Mathisen J (1987) The early development of neurons with GABA immunoreactivity in CNS of Xenopus laevis embryos. J Comp Neurol 261:435–449
Robertson RM, Wisniowski L (1988) GABA-like immunoreactivity of identified interneurons in the flight system of the locust, Locusta migratoria. Cell Tissue Res 254:331–340
Schultze LS (1899) Die Regeneration des Ganglions von Ciona intestinalis L. und über das Verhältnis der Regeneration und Knospung zur Keimenblätterlehre. Jena Zeitung Naturwiss 33:263–344
Thorndyke MC, Georges D (1988) Functional aspects of peptide neurohormones in protochordates. In: Thorndyke MC, Goldsworthy GJ (eds) Neurohormones in invertebrates. Cambridge University Press, Cambridge, pp 235–258
Vitellaro-Zuccarello L, De Biasi S (1988) GABA-like immunoreactivity in the pedal ganglia of Mytilus galloprovincialis: light and electron microscopic study. J Comp Neurol 267:516–524
Welsh JH, Loveland RE (1968) 5-Hydroxytryptamine in the ascidian Ciona intestinalis. Comp Biochem Physiol 27:719–722
Yoshida M, Nogi H, Tani Y (1987) Nervous mechanisms of spawning in regular echinoids. In: Ali MA (ed) Nervous systems in invertebrates. Plenum Press, New York London, pp 559–572
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bollner, T., Beesley, P.W. & Thorndyke, M.C. Distribution of GABA-like immunoreactivity during post-metamorphic development and regeneration of the central nervous system in the ascidian Ciona intestinalis . Cell Tissue Res 272, 553–561 (1993). https://doi.org/10.1007/BF00318562
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318562