Summary
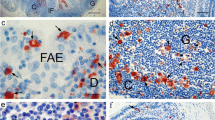
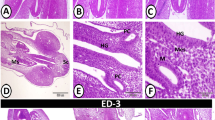
The zonulae occludentes of the dome epithelia and adjacent non-dome epithelia in four locations of the gut-associated lymphoid tissue (GALT) in the rabbit ileum and caecum (Peyer's patches, sacculus rotundus, caecal lymphoid patches, appendix) were studied in freeze-fracture replicas. In all locations the zonulae occludentes of the dome epithelium are composed of more junctional strands than in the corresponding non-dome epithelium. In the dome epithelia of Peyer's and caecal lymphoid patches the zonulae occludentes show considerable structural variation; the number of superimposed strands is ∼10 (range 5–18). In the dome epithelia of sacculus rotundus and appendix, in addition to zonulae occludentes, extended networks of junctional strands (fasciae occludentes) are present particularly between M-cells and enterocytes. The zonulae occludentes consist of ∼8 to 9 (range 5–15) superimposed strands; the fasciae occludentes extend up to a depth of 20μm on the lateral membranes. The presence of the fasciae occludentes correlates with the appearance of regularly shaped clusters of lymphocytes, which are most developed in the dome epithelia of sacculus rotundus and appendix. These results suggest (1) that in contrast to the dome epithelia of Peyer's and caecal lymphoid patches those of sacculus rotundus and appendix are compartmentalized, and (2) that the mobility of lymphocytes and diffusion of antigens in the dome epithelia of sacculus rotundus and appendix is restricted.
Similar content being viewed by others
References
Bockman DE, Cooper MD (1973) Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix and Peyer's patches. Am J Anat 136:455–478
Carson JL, Collier AM, Hu SCS (1980) Ultrastructural studies of hamster tracheal epithelium in vivo and in vitro. J Ultrastruct Res 70:70–81
Claude P (1978) Morphological factors influencing transepithelial permeability: a model for the resistance of the zonulae occludens. J Membr Biol 39:219–232
Claude P, Goodenough DA (1973) Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol 58:390–400
DiBona DR (1985) Functional analysis of tight junction organization. Pflügers Arch 405:S59-S66
Ducroc R, Heyman M, Beaufrere B, Morgat JL, Desjeux JF (1983) Horseradish peroxidase transport across rabbit jejunal and Peyer's patches in vitro. Am J Physiol 245:G54-G58
Farquhar MG, Palade GE (1963) Junctional complexes in various epithelia. J Cell Biol 17:375–412
Frömter E, Diamond J (1972) Route of passive ion permeation in epithelia. Nature 235:9–13
Gebert A (1988) Die Zellverbindungen in den Epithelien der Darmassozierten lymphatischen Gewebe vom Kaninchen. Med Diss, Hannover
Gebert A, Bartels H (1987) Gefrierbruchaspekte des Follikel-assoziierten Epithels (FAE) der Peyer-Plaques. Verh Anat Ges 81:769–770
Hull BE, Staehelin LA (1976) Functional significance of the variations in the geometrical organisation of tight junction networks. J Cell Biol 68:688–704
Kachar B, Pinto da Silva P (1981) Rapid massive assembly of tight junction strands. Science 213:541–543
Karnovsky MJ (1971) Use of ferrocyanide-reduced osmium tetroxide in electron microscopy. Proc 11th Annual Meeting Am Soc Cell Biol, New Orleans, p 146
Knutton S, Limbrick AR, Robertson JD (1978) Structure of occluding junctions in ileal epithelial cells of suckling rats. Cell Tissue Res 191:449–462
Luciano L, Castellucci M, Reale E (1981) The brush cells of the common bile duct of the rat. Thin section, freeze fracture and scanning electron microscopy. Cell Tissue Res 218:403–420
Luciano L, Reale E, Rechkemmer G, Engelhardt W von (1984) Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and the distal colon of guinea pig. J Membrane Biol 82:145–156
Madara JL (1987) Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol 253:C171-C175
Madara JL (1990) Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangements of tight junction. J Membrane Biol 116:177–184
Madara JL, Trier JS (1982) Structure and permeability of goblet cell tight junctions in rat small intestine. J Membrane Biol 66:145–157
Madara JL, Trier JS, Neutra MR (1980) Structural changes in the plasma membrane accompanying differentiation of epithelial cells in human and monkey small intestine. Gastroenterology 78:963–975
Madara JL, Neutra MR, Trier JS (1981) Junctional complexes in fetal rat small intestine during morphogenesis. Dev Biol 86:170–178
Madara JL, Bye WA, Trier JS (1984) Structural features of and cholesterol distribution in M-cell membranes in guinea pig, rat, and mouse Peyer's patches. Gastroenterology 87:1091–1103
Magnusson T, Jacobson JB, Stackpole CW (1978) Relationship between intercellular permeability and junction organization in the preimplanation mouse embryo. Dev Biol 67:214–224
Martinez-Palomo A, Erlij D (1975) Structure of tight junctions in epithelia with different permeability. Proc Natl Acad Sci USA 72:4487–4491
Mazariegos MR, Tice LW, Hand AR (1984) Alteration of tight junctional permeability in the rat parotid gland after isoproterenol stimulation. J Cell Biol 98:1865–1877
McNutt NS, Weinstein RS (1973) Membrane ultrastructure at mammalian intercellular junctions. Prog Bioph Mol Biol 26:42–101
Meyer RA, McGinley D, Posalaky Z (1986) Effects of aspirin on tight junction structure of the canine gastric mucosa. Gastroenterology 91:351–359
Montesano R, Gabbiani G, Perrelet A, Orci L (1976) In vivo induction of tight junction proliferation in rat liver. J Cell Biol 68:793–798
Muthmann E (1913) Beiträge zur vergleichenden Anatomie des Blinddarmes und lymphoiden Organe des Darmkanals bei Säugetieren und Vögeln. Anat Hefte 1. Abt, 8:65–114
Neutra MR, Phillips TL, Mayer EL, Fishkind DJ (1987) Transport of membrane-bound macromolecules by M-cells in follicle-associated epithelium of rabbit Peyer's patches. Cell Tissue Res 247:537–546
Nieuwenhuis P (1971) The rabbit appendix: a central or peripheral lymphoid organ? Adv Exp Med Biol 12:25–30
Owen RL (1977) Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology 72:440–451
Owen RL, Bhalla DK (1983) Lymphoepithelial organs and lymph nodes. Biomed Res Applications SEM 3:79–169
Owen RL, Jones AL (1974) Epithelial cell specialisation within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 66:189–203
Owen RL, Apple RT, Bhalla DK (1986) Morphometric and cytochemical analysis of lysosomes in rat Peyer's patch follicle epithelium: their reduction in volume fraction and acid phosphatase content in M cells compared to adjacent enterocytes. Anat Rec 216:521–527
Pappo J, Steger HJ, Owen RL (1988) Differential adherens of epithelium overlying gut-associated lymphoid tissue. An ultrastructural study. Lab Invest 58:692–697
Pitelka DR, Taggart BN (1983) Mechanical tension induces lateral movements of intramembrane components of the tight junctions: Studies on mouse mammary cells in culture. J Cell Biol 96:606–612
Rassat J, Robenek H, Themann H (1981) Ultrastructural changes in mouse hepatocytes exposed to vinblastin sulfate with special reference to the intercellular junctions. Eur J Cell Biol 24:203–210
Reese TS, Karnovsky MJ (1967) Fine structure localisation of a blood-brain barrier to exogenous peroxidase. J Cell Biol 34:207–217
Rhodes RS, Karnovsky MJ (1971) Loss of macromolecular barrier function associated with surgical trauma to the intestine. Lab Invest 25:220–229
Robenek H, Döldissen M, Themann H (1980) Morphological changes of tight junctions in the rat liver after chronic administration of N-nitrosomorpholine (NNM) as revealed by freeze-fracturing. J Ultrastruct Res 70:82–91
Roy MJ, Ruiz A (1986) Dome epithelial M cells dissociated from gut-associated lymphoid tissues. Am J Vet Res 12:2577–2583
Schmedtje JF (1980) Lymphocyte positions in the dome epithelium of the rabbit appendix. J Morphol 166:179–195
Schneeberger EE (1976) Ultrastructural basis for aleolar-capillary permeability to protein. In: Lung Liquids, Ciba Foundation Symp 38, London, pp 3–28
Schneeberger EE (1980) Heterogeneity of tight junction morphology in extrapulmonary and intrapulmonary airways of the rat. Anat Rec 198:193–208
Sicinski P, Rowinski J, Warchol JB, Jarzabek Z, Gut W, Szczygiel B, Bielecki K, Koch G (1990) Poliovirus type 1 enters the human host through intestinal M-cells. Gastroenterology 98:56–58
Snipes RL (1978) Anatomy of the rabbit cecum. Anat Embryol 155:57–80
Staehelin LA (1974) Structure and function of intercellular junctions. Int Rev Cytol 39:191–283
Staehelin LA, Mukherjee TM, Williams WW (1969) Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma 67:165–184
Teillet MA, Hugon JS, Calvert R (1981) The occluding junctions of mouse duodenal enterocytes during development. Cell Tissue Res 217:65–77
Walker RI, Porvaznik MJ (1978) Disruption of the permeability barrier (zonula occludens) between intestinal epithelial cells by lethal doses of endotoxin. Infect Immun 21:655–658
Wekerle H, Ketelsen UP, Ernst M (1980) Thymic nurse cells, lymphoepithelial cell complexes in murine thymuses: morphological and serological characterisation. J Exp Med 151:925–944
Weltzin R, Lucia-Jandris P, Michetti P, Fields BN, Kraehenbuhl JP, Neutra MR (1989) Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol 108:1673–1685
Wolf JL, Bye WA (1984) The membranous epithelial (M) cell and the mucosal immune system. Ann Rev Med 35:95–112
Wolf JL, Rubin DH, Finberg R, Kauffman RS, Sharpe AH, Trier JS, Fields BN (1981) Intestinal M-cells: a pathway for entry of reovirus into the host. Science 212:471–472
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gebert, A., Bartels, H. Occluding junctions in the epithelia of the gut-associated lymphoid tissue (GALT) of the rabbit ileum and caecum. Cell Tissue Res 266, 301–314 (1991). https://doi.org/10.1007/BF00318186
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318186