Abstract
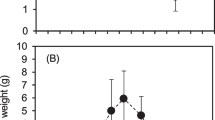
The decline and disappearance of Littorella uniflora from oligotrophic waters which have become eutrophic has been associated with shading or reduced CO2 supply. However NO sup−inf3 concentrations can reach very high levels (100–2000 mmol m−3 compared with <1–3 in oligotrophic habitats). To investigate the impact of NO sup−inf3 loading alone, plants were grown under three NO sup−inf3 regimes (very low, near-natural and high). The interactive effects of NO sup−inf3 and photon flux density (low and high regimes) on N assimilation and accumulation, CO2 concentrating mechanisms, C3 photosynthesis and growth were also examined. The results were unexpected. Increased NO sup−inf3 supply had very little effect on photosynthetic capacity, crassulacean acid metabolism (CAM) or lacunal CO2 concentrations ([CO2]i), although there was considerable plasticity with respect to light regime. In contrast, increased NO sup−inf3 supply resulted in a marked accumulation of NO sup−inf3 , free amino acids and soluble protein in shoots and roots (up to 25 mol m−3, 30 mol m−3 and 9 mg g−1 fresh weight respectively in roots), while fresh weight and relative growth rate were reduced. Total N content even under the very low NO sup−inf3 regime (1.6–2.3%) was mid-range for aquatic and terrestrial species (and 3.1–4.3% under the high NO sup−inf3 regime). These findings, together with field data, suggest that L. uniflora is not growth limited by low NO sup−inf3 supply in natural oligotophic habitats, due not to an efficient photosynthetic nitrogen use but to a slow growth rate, a low N requirement and to the use of storage to avoid N stress. However the increased NO sup−inf3 concentrations in eutrophic environments seem likely have detrimental effects on the long-term survival of L. uniflora, possibly as a consequence of N accumulation.
Similar content being viewed by others
References
Aerts R (1989) Nitrogen use efficiency in relation to nitrogen availability and plant community composition. In: Lambers H (ed) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 285–297
Anderson JM, Andersson B (1988) The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem Sci 13:351–355
Arts GHP, Van der Velde G, Roelofs JGM, Van Sway CAM (1990) Successional changes in the soft-water macrophyte vegation of (sub)atlantic sandy lowland regions during this century. Fresh-water Biol 24:287–294
Bailey-Watts AE (1988) Studies on the control of the early spring diatom maximum in Loch Leven in 1981. In: Round RE (ed) Algae and the aquatic environment. Biopress, Bristol, pp 53–87
Beer S, Sand-Jensen K, Madsen TV, Nielsen SL (1991) The carboxylase activity of Rubisco and the photosynthetic performance in aquatic plants. Oecologia 87:429–434
Berendse F, Aerts R (1987) Nitrogen-use-efficiency: a biologically meaningful definition? Funct Ecol 1:293–296
Bjorkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Zeigler H (eds) Encyclopedia of Plant Physiology, new series vol 12A. Springer, Berlin Heidelberg New York, pp 57–107
Blom-Zanstra M, Lampe JEM (1985) The role of nitrate in the osmoregulation of lettuce (Lactuca sativa L.) grown at different light intensities. J Exp Bot 36:1043–1052
Borland AM, Griffiths H (1989) The regulation of citric acid accumulation and carbon recycling during CAM in Ananas comosus. J Exp Bot 40:53–60
Boston HL, Adams MS (1985) Seasonal diurnal acid rythms in two aquatic crassulacean acid metabolism plants. Oecologia 65:573–579
Boston HL (1986) A discussion of the adaptation for carbon acquisition in relation to the growth strategy of aquatic isoetids. Aquat Bot 26:259–270
Boston HL, Adams MS (1987) Productivity growth and photosynthesis of two small “isoetid” plants Littorella uniflora and Isoetes macrospora. J Ecol 75:333–350
Boston HL, Adams MS, Pienkowski TP (1987) Utilization of sediment CO2 by selected North American isoetids. Ann Bot 60:485–494
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown RH (1978) A difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Sci 18:93–98
Carrick TR, Sutcliffe DW (1982) Concentrations of major ions in takes of the English Lake District (1953–1978). Occasional publication No 16. Freshwater Biological Association. The Ferry House, Ambleside, Cumbria, UK
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Chapin FS, Kedrowski RA (1983) Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64:176–191
Chapin FS, Schulze E-D, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Christiansen R, Friis NJS, Søndergaard M (1985) Leaf production and nitrogen and phosphorus tissue content of Littorella uniflora (L.) Aschers. in relation to nitrogen and phosphorus enrichment of the sediment in oligotrophic lake Hampden, Denmark. Aquat Bot 23 1–11
Clapham AR, Tutin RG, Warburg GF (1981) Excursion flora of the British Isles. Cambridge University Press, Cambridge
Den Hartog C, Segal C (1964) A new classification of the water-plant communities. Acta Bot Neerl 13:367–393
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs consequences and control. In: Briggs WR (ed) Photosynthesis. Liss, New York, pp 183–205
Farmer AM, Spence DHN (1986) The growth strategies and distribution of isoetids in Scottish freshwater lochs. Aquat Bot 26:247–258
Farmer AM, Maberly SC, Bowes G (1986) Activities of carboxylation enzymes in freshwater macrophytes. J Exp Bot 37:1568–1573
Fichtner K, Quick WP, Schulze E-D, Mooney HA, Rodermel SR, Bogorad L, Stitt M (1993) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with “antisense” rbcS. V. Relationship between photosynthetic rate, storage strategy, biomass allocation and vegetative plant growth at three different nitrogen supplies. Planta 190:1–9
Field C, Mooney HA (1986) The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Franco AC, Ball E, Lüttge U (1991) The influence of nitrogen light and water stress on CO2 exchange and organic acid accumulation in the tropical C3-CAM tree Clusia minor. J Exp Bot 42:597–603
Gebauer G, Rehder H, Wollenweber B (1988) Nitrate, nitrate reduction and organic nitrogen in plants from different ecological and taxonomic groups of Central Europe. Oecologia 75:371–385
Gerloff GC, Krombholz PH (1966) Tissue analysis as a measure of nutrient availability for the growth of angiosperm aquatic plants. Limnol Oceanogr 11:529–537
Goulder R, Boatman DJ (1971) Evidence that nitrogen supply influences the distribution of a freshwater macrophyte Ceratophyllum demersum. J Ecol 783–791
Grahn O (1977) Macrophyte succession in Swedish lakes caused by deposition of airborne acid substances. Water Air Soil Pollut 7:295–305
Griffiths H (1989) Carbon dioxide concentrating mechanisms and the evolution of CAM in vascular epiphytes. In: Lüttge U (ed) Evolution and ecophysiology of vascular plants as epiphytes (Ecological Studies 76). Springer, Berlin Heidelberg New York, pp 42–86
Griffiths H, Ong BL, Avadhani PN, Goh CJ (1989) Recycling of respiratory CO2 during crassulacean acid metabolism: alleviation of photoinhibition in Pyrrosia piloselloides. Planta 179:115–122
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
Groenhof AC, Smirnoff N, Bryant JA (1988) Enzymic activities asociated with the ability of aerial and submerged forms of Littorella uniflora (L) Aschers to perform CAM. J Exp Bot 39:353–361
Hoagland DR, Snyder WC (1932) Nutrition of strawberry plant under controlled conditions: (a) effects of deficiencies of boron and certain other elements; (b) susceptibility to injury from sodium salts. Proc Am Soc Hort Sci 30:288–293
Holden AV, Cains LA (1973) Nutrient chemistry of Loch Leven Kinross. Proc R Soc Edinb B 74:101–121
Hostrup O, Wiegleb G (1991) Anatomy of leaves of submerged and emergent forms of Littorella (L.) Ascherson. Aquat Bot 39:195–209
Jupp BP, Spence DHN (1977) Limitations on macrophytes in a eutrophic lake, Loch Leven. J Ecol 65:175–186
Killingbeck KT (1993) Inefficient nitrogen resorption in genets of the actinorhizal nitrogen fixing shrub Comptonia peregrine; physiological ineptitude or evolutionary tradeoff? Oecologia 94:542–549
Ku MB, Schmitt MR, Edwards GE (1979) Quantitative determination of RußP Carboxylase-oxygenase protein in leaves of several C3 and C4 plants. J Exp Bot 30:89–98
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 23:187–261
Levitt J (1972) Responses of plants to environmental stresses. Academic Press, New York
Lodge DM (1991) Herbivory on freshwater macrophytes. Aquat Bot 41:195–224
Lüttge U (1993) The role of crassulaean acid metabolism (CAM) in the adaptation of plants to salinity. New Phytol 125:59–71
Mackereth FJH, Heron J, Talling JF (1978) Water analysis (Scientific Publication 36). Freshwater Biological Association, The Ferry House, Ambleside, Cumbria, UK
Madsen TV (1987a) Sources of inorganic carbon acquired through CAM in Littorella uniflora (L.) Aschers. J Exp Bot 38:367–377
Madsen TV (1987b) The effect of different growth conditions on dark and light carbon assimilation in Littorella uniflora. Physiol Plant 70:183–188
Melzer A, Exler D (1982) Nitrate and nitrite-reductase activities in aquatic macrophytes. In: Symoens JJ, Hoper SS, Compere P (eds) Studies on aquatic vascular plants. Royal Botanical Society of Belgium, Brussels, pp 128–135
Millard P (1988) The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ 11:1–8
Mohr H, Neininger A Seith B (1992) Control of nitrate reductase and nitrite reductase gene expression by light, nitrate and a plastidic factor. Bot Acta 105:81–89
Newman RM (1991) Herbivory and detritivory on freshwater macrophytes by invertebrates: a review. J North Am Benthol Soc 10:89–114
Nielsen SL, Sand-Jensen K (1989) Regulation of photosynthetic rates of submerged rooted macrophytes. Oecologia 81:364–368
Nielsen SL, Sand-Jensen K (1991) Variation in growth rates of submerged rooted macrophytes. Aquat Bot 39:109–120
Nobel PS (1983) Nutrient levels in cacti-relation to nocturnal acid accumulation and growth. Am J Bot 70:1244–1253
Osborne BA, Whittington WJ (1981) Eco-physiological aspects of interspecific and seasonal variation in nitrate utilization in the genus Agrostis. New Phytol 87:595–614
Osmond CB (1983) Interactions between irradiance, nitrogen nutrition and water stress in the sun-shade responses of Solanum dulcamara. Oecologia 57:316–321
Ostrofsky ML, Zettler ER (1986) Chemical defences in aquatic plants. J Ecol 74:279–287
Paul MJ, Cockburn W (1980) The stimulation of CAM activity in mesembryanthemun crystallinum in nitrate- and phosphatedeficient conditions. New Phytol 114:391–398
Pearsall WH (1921) The development of vegetation in the English Lakes considered in relation to the general evolution of glacial lakes and rock basins. Proc R Soc B 92:259–284
Phillips GL, Eminson D, Moss B (1978) A mechanism to account for macrophyte decline in progressively entrophicated freshwaters. Aquat Bot 4:103–126
Quick WP, Fichtner K, Schulze E-D, Wendler R, Leegood RC, Mooney H, Rodermel SR, Bogorad L, Stitt M (1992) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with “antisense” rbcS. IV. Impact on photosynthesis in conditions of altered nitrogen supply. Planta 188:522–531
Raven JA (1984) Energetics and transport in aquatic plants (M.B.L. Lectures in Biology vol 4). Liss, New York
Raven JA (1985) Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy nitrogen and water. New Phytol 101:25–77
Raven JA, Osmond CB (1992) Inorganic C acquisition processes and their ecological significance in inter-and sub-tidal macroalgae of North Carolina. Funct Ecol 6:41–47
Raven JA, Smith FA (1976) Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytol 76:415–431
Raven JA, Handley LL Macfarlane JJ, McInroy S, McKenzie L, Richards JH Samuelsson G (1988) The role of CO2 uptake by roots and CAM in acquisition of inorganic C by plants of the isoetid life-form: a review with new data on Eriocaulon decangulare L. New Phytol 108:157–191
Redinbaugh MG, Campbell WH (1991) Higher plant responses to environmental nitrate. Physiol Plant 82:640–650
Richardson K, Griffiths H, Reed H, Raven JA, Griffiths NM (1984) Inorganic carbon assimilation in the isoetids Isoetes lacustris L. and Lobelia dortmanna L. Oecologia 61:115–121
Robe WE (1989) Ecophysiology of Littorella uniflora: interactions between inorganic carbon supply and C3 and CAM photosynthetic characteristics. Ph.D Thesis, University of Newcastle upon Tyne
Robe WE, Griffiths H (1988) C3 and CAM photosynthetic characteristics of the submerged aquatic macrophyte Littorella uniflora: regulation of leaf internal CO2 supply in response to variation in rooting substrate inorganic carbon concentration. J Exp Bot 39:1397–1410
Robe WE, Griffiths H (1990) Photosynthesis of Littorella uniflora grown under two PAR regimers: C3 and CAM gas exchange and the regulation of internal CO2 and O2 concentraions. Oecologia 85:128–136
Robe WE, Griffiths H (1992) Seasonal variation in the ecophysiology of Littorella uniflora (L.) Ascherson in acidic and eutrophic habitats. New Phytol 120:289–304
Roelofs JGM, (1983) Impact of acidification and eutrophication on macrophyte communities in soft waters in the Netherlands. I. Field observations. Aquat Bot 17:139–155
Roelofs JGM, Schuurkes JAAR, Smits AJM (1984) Impact of acidification and eutrophication on macrophyte communities in soft waters. II. Experimental studies. Aquat Bot 18:389–411
Rosnitschek-Schimmel I (1985) Seasonal dynamics of nitrogenous compounds in a nitrophilic weed I. Changes in inorganic and organic nitrogen fractions of the different plant parts of Urtica dioica. Plant Cell Physiol 26:169–176
Sand-Jensen K, Borum J (1984) Epiphyte shading and its effect on photosynthesis and diel metabolism of Lobelia dortmanna L. during the spring bloom in a Danish lake. Aquat Bot 20:109–119
Sand-Jensen K, Søndergaard M (1979) Distribution and quantitative development of aquatic macrophytes in relation to sediment characteristics in oligotrophic Lake Kalgaard, Denmark. Freshwater Biol 9:1–11
Sand-Jensen K, Søndergaard M (1981) Phytoplankton and eipiphyte development and their shading effect on submerged macrophytes in lakes of different nutrient status. Int Rev Ges Hydrobiol Hydrogr 66:529–552
Santos I, Salema R (1991) Nitrogen nutrition and the level of crassulacean acid metabolism in Kalanchoe lateritia Engl. Plant Cell Environ 14:311–317
Schmitt MR, Edwards GE (1981) Photosynthetic capacity and nitrogen use efficiency of maize, wheat and rice: a comparison between C3 and C4 photosynthesis. J Exp Bot 32:459–466
Schuurkes JAAR (1987) Acidification of surface waters by atmospheric deposition. Thesis, Nijmegen
Schuurkes JAAR, Kok CJ, Den Hartog C (1986) Ammonium and nitrate uptake by aquatic plants from poorly buffered and acidified water. Aquat Bot 24:131–146
Seddon B (1965) Occurrence of Isoetes echinospora in eutrophic lakes in Wales. Ecology 46:747–748
Smith WG, Boston HL, Adams MS (1985) A preliminary study of the source and fate of carbon acquired via CAM in Littorella uniflora var. americana (Fern.) Gl. J Freshwater Ecol 3:203–209
Spence DHN (1964) The macrophyte vegetation of freshwater locks swamps and associated fens. In: Burnett JJ (ed) The vegetation of Scotland. Oliver and Boyd, Edinburgh, pp 306–381
Spence DHN (1967) Factors controlling the distribution of freshwater macrophytes with particular reference to the lochs of Scotland. J Ecol 55:147–170
Stewart GR, Lee JA, Orebamjo TO (1973) Nitrogen metabolism in halophytes. II. Nitrate availability and utilization. New Phytol 72:539–546
Stitt M, Schulze D (1994) Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ 17:465–487
Talling JF, Heaney SI (1983) Evaluation of historical data on the nutrient status of Esthwaite Water, Cumbria. Freshwater Biological Association, The Ferry House, Ambleside, Cumbria, UK
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (1964–1988) Flora Europaea. Cambridge University Press, Cambridge
Van Dam H (1988) Acidification of three moorland pools in The Netherlands by acid precipitation and extreme drought periods over seven decades. Freshwater Biol 20:157–176
Vernon LP, Seely GR (1966) The chlorophylls. Academic Press, New York
Werf A van der, Visser AJ, Schieving F, Lambers H (1993) Evidence for optimal partitioning of biomass and nitrogen at a range of nitrogen availability in a fast and slow growing species. Funct Ecol 7:63–74
Westlake DF (1982) The primary productivity of aquatic plants. In: Symoens JS, Hoper SS, Compere P (eds) Studies on aquatic vascular plants. Royal Society of Belgium, Brussels, pp 165–180
Widmann J, Gebauer G, Rehder H, Ziegler H (1993) Biomass production and nitrogen contents of the CAM plants Kalanchoe daigremontiana and K. tubiflora in cultures with different nitrogen and water supply. Oecologia 82:478–483
Winter K, Foster JG, Schmitt MR, Edwards GE (1982) Activity and quantity of ribulose bisphosphate carboxylase- and phosphoenolpyruvate carboxylase-protein in two crassulacean acid metabolism plants in relation to leaf age, nitrogen nutrition, and point in time during a day/night cycle. Planta 154:309–317
Woodin SJ, Lea JA (1987) The effects of nitrate ammonium and temperature on nitrate reductase activity in Sphagnum species. New Phytol 105:103115
Yemm EW, Cocking EC (1955) The determination of amino-acids with ninhydrin. Biol Chem 80:209–213
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robe, W.E., Griffiths, H. The impact of NO sup−inf3 loading on the freshwater macrophyte Littorella uniflora: N utilization strategy in a slow-growing species from oligotrophic habitats. Oecologia 100, 368–378 (1994). https://doi.org/10.1007/BF00317857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317857