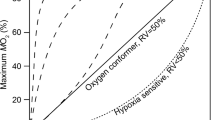
Summary
The standard metabolic rate (SMR) and critical O2 tension (Pc) of water-breathing mudpuppies and hellbenders were determined at 20° C using open-system respirometry. Both species are metabolic O2 regulators, although the Pc of hellbenders (90 mmHg) is much higher than that of mudpuppies (40 mmHg). The SMR of the two species in water saturated with air was similar (19.5 and 20.0 μl O2/g·h for Cryptobranchus and Necturus, respectively) and not different from that of salamanders in general. Both species were able to survive for at least 5–11 days in severely hypoxic water (9–10 mmHg) by breathing air, indicating that the lungs are functional accessory respiratory structures.
We conclude that hellbenders are restricted to relatively cool and flowing waters because of their limited gas exchange capabilities, particularly with regard to their limited aerobic scope for activity and slow recovery from exercise. Necturus maculosus is much more tolerant of hypoxia, but it is not known if they can inhabit areas were hypoxia is combined with hypercarbia.
Similar content being viewed by others
References
Baker CL (1949) The comparative anatomy of the aortic arches of the urodeles and their relation to respiration and degree of metamorphosis. J Tenn Acad Sci 24:12–40
Beffa DA (1976) Responses of Cryptobranchus alleganiensis alleganiensis to different oxygen concentrations, temperatures and photoperiod. M.S. Thesis, Southwest Missouri State University, Springfield, Missouri, p 37
Boutilier RG, McDonald DG, Toews DP (1980) The effects of enforced activity on ventilation, circulation and blood acid-base balance in the aquatic gill-less urodele, Cryptobranchus alleganiensis; a comparison with the semi-terrestrial anuran, Bufo marinus. J Exp Biol 84:289–302
Boutilier RG, Toews DP (1981a) Respiratory properties of blood in a strictly aquatic and predominantly skin-breathing urodele, Cryptobranchus alleganiensis. Respir Physiol 46:161–176
Boutilier RG, Toews DP (1981b) Respiratory, circulatory and acidbase adjustments to hypercapnia in a strictly aquatic and predominantly skin-breathing urodele, Cryptobranchus alleganiensis. Respir Physiol 46:177–192
Brown NR (1985) Changes in LDH activity in Cryptobranchus due to imposed environmental stress. M.S. Thesis, Southwest Missouri State University, Springfield, Missouri, p 33
Burggren WW, Feder ME (1986) Effect of experimental ventilation of the skin on cutaneous gas exchange in the bullfrog. J Exp Biol 121:445–449
Burggren WW, Moalli R (1984) ‘Active’ regulation of cutaneous gas exchange by capillary recruitment in amphibians: experimental evidence and a revised model for skin respiration. Respir Physiol 55:379–392
Conant R (1975) A field guide to reptiles and amphibians of eastern and central North America. Houghton Mifflin Co., Boston
Darnell RM (1949) The aortic arches and associated arteries of caudate Amphibia. Copeia 1949:18–31
Feder ME (1976) Lunglessness, body size, and metabolic rate in salamanders. Physiol Zool 49:398–406
Feder ME, Burggren WW (1985) Cutaneous gas exchange in vertebrates: design, patterns, control and implications. Biol Rev 60:1–45
Gatten RE, Miller K, Full R (1990) Energetics of amphibians at rest and during locomotion. In: Feder ME, Burggren WW (eds) Environmental Physiology of the Amphibia. University of Chicago Press, Chicago, Illinois. (In press)
Guimond RW, Hutchison VH (1972) Pulmonary, branchial and cutaneous gas exchange in the mudpuppy, Necturus maculosus maculosus (Rafinesque). Comp Biochem Physiol 42A:367–392
Guimond RW, Hutchison VH (1973) Aquatic respiration: an unusual strategy in the hellbender Cryptobranchus alleganiensis alleganiensis (Daudin). Science 182:1263–1265
Guimond RW, Hutchison VH (1976) Gas exchange of the giant salamanders of North America. In: Hughes GM (ed) Respiration of Amphibious Vertebrates, pp 313–338. Academic Press, New York
Harlan RA, Wilkinson RF (1981) The effects of progressive hypoxia and rocking activity on blood oxygen tension for hellbenders, Cryptobranchus alleganiensis. J herpetol 15:383–387
Harris JP (1959) The natural history of Necturus: I. Habitats and habits. Field Laborat 27:11–20
Heisler N, Forcht G, Ultsch GR, Anderson JF (1982) Acid-base regulation in response to environmental hypercapnia in two aquatic salamanders, Siren lacertina and Amphiuma means. Respir Physiol 49:141–158
Lenfant C, Johansen K (1967) Respiratory adaptations in selected amphibians. Respir Physiol 2:247–260
McCutcheon FH, Hall FG (1937) Hemoglobin in the amphibia. J Cell Comp Physiol 9:191–197
Miller K, Hutchison VH (1979) Activity metabolism in the mudpuppy, Necturus maculosus. Physiol Zool 52:22–37
Moalli R, Meyers RS, Ultsch GR, Jackson DC (1981) Acid-base balance and temperature in a predominantly skin-breathing salamander Cryptobranchus alleganiensis. Respir Physiol 43:1–11
Nickerson MA, Mays CE (1973) A study of the Ozark hellbender Cryptobranchus alleganiensis bishopi. Ecology 54:1164–1165
Noble GK (1925) The integumentary, pulmonary, and cardiac modifications correlated with increased cutaneous respiration in the amphibia: a solution of the ‘hairy frog’ problem. J Morphol Physiol 40:341–416
Ott ME, Heisler N, Ultsch GR (1980) A re-evaluation of the relationship between temperature and the critical oxygen tension in freshwater fishes. Comp Biochem Physiol 67A:337–340
Putnam JL, Dunn JF (1978) Septation in the ventricle of the heart of Necturus maculosus. Herpetologica 34:292–297
Putnam JL, Parkerson JB (1985) Anatomy of the heart of the amphibia II. Crytobranchus alleganiensis. Herpetologica 41:287–298
Romer AS, Parsons TS (1986) The vertebrate body. Saunders College Publishing, New York
Shield JW, Bentley PJ (1973) Respiration of some urodele and anuran amphibia — I. In water, role of the skin and gills. Comp Biochem Physiol 46A, 17–28
Steffenson JF (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol Biochem 64:49–59
Stiffler DF, Tufts BL, Toews DP (1983) Acid-base and ionic balance in Ambystoma tigrinum and Necturus maculosus during hypercapnia. Am J Physiol 245:R689-R694
Ultsch GR (1973a) A theoretical and experimental investigation of the relationships between metabolic rate, body size, and oxygen exchange capacity. Respir Physiol 18:143–160
Ultsch GR (1973b) The effects of water hyacinths (Eichhornia crassipes) on the microenvironment of aquatic communities. Archiv Hydrobiol 72:460–473
Ultsch GR (1976a) Eco-physiological studies studies of some metabolic and respiratory adaptations of sirenid salamanders. In: Hughes GM (ed) Respiration of Amphibious Vertebrates, pp 287–312. Academic Press, New York
Ultsch GR (1976b) Respiratory surface area as a factor controlling the standard rate of O2 consumption of aquatic salamanders. Respir Physiol 26:357–369
Ultsch GR, Anderson JF (1988) Gas exchange during hypoxia and hypercarbia of terrestrial turtles: a comparison of a fossorial species (Gopherus polyphemus) with a sympatric nonfossorial species (Terrapene carolina). Physiol Zool 61:142–152
Ultsch GR, Jackson DC, Moalli R (1981) Metabolic oxygen conformity among lower vertebrates: the toadfish revisited. J Comp Physiol 142:439–443
Wakeman JM, Ultsch GR (1975) The effects of dissolved O2 and CO2 on metabolism and gas exchange partitioning in aquatic salamanders. Physiol Zool 48:348–359
Weber RE, Wells RMG, Rossetti JE (1985) Adaptations to neoteny in the salamander, Necturus maculosus. Blood respiratory properties and interactive effects of pH, temperature, and ATP on hemoglobin oxygenation. Comp Biochem Physiol 80A:495–501
Yeager DP, Ultsch GR (1989) Physiological regulation and conformation: A BASIC program for the determination of critical points. Physiol Zool 62:888–907
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ultsch, G.R., Duke, J.T. Gas exchange and habitat selection in the aquatic salamanders Necturus maculosus and Cryptobranchus alleganiensis . Oecologia 83, 250–258 (1990). https://doi.org/10.1007/BF00317760
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317760