Abstract
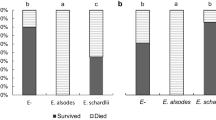
Several species of willow leaf beetles use hostplant salicin to produce a defensive secretion that consists of salicylaldehyde. Generalist arthropod predators such as ants, ladybird beetles, and spiders are repelled by this secretion. The beetle larvae produce very little secretion when they feed on willows that lack salicylates, and salicin-using beetles prefer salicylate-rich willows over salicylate-poor ones. This preference may exist because the larvae are better defended against natural enemies on salicylate-rich willows. If this is true, the larvae should survive longer on those willows in nature. However, this prediction has not been tested. I determined the larval growth and survival of Chrysomela aeneicollis (Coleoptera: Chrysomelidae) on five willow species (Salix boothi, S. drummondiana, S. geyeriana, S. lutea, and S. orestera). These species differed in their salicylate chemistries and in leaf toughness but not in water content. The water content varied among the individual plants. Larval growth of C. aeneicollis did not differ among the five species in the laboratory, but it varied among the individual plants and it was related to the water content. In the field, C. aeneicollis larvae developed equally rapidly on the salicylate-poor S. lutea and on the salicylate-rich S. orestera. Larval survival was greater on S. orestera than on S. lutea in one year (1986), but there was no difference between them during three succeeding years. In another survivorship experiment, larval survival was low on the medium-salicylate S. geyeriana, but high on the salicylate-poor S. boothi and on S. orestera. Larval survival in the field was related to the larval growth and water content that had been previously measured in the laboratory. These results showed that the predicted relationship between the host plant chemistry and larval survival did not usually exist for C. aeneicollis. One possible reason for this was that the most important natural enemies were specialist predators that were unaffected by the host-derived defensive secretion. One specialist predator, Symmorphus cristatus (Hymenoptera: Eumenidae), probably caused much of the mortality observed in this study. I discuss the importance of other specialist predators to salicin-using leaf beetles.
Similar content being viewed by others
References
Berland L (1928) Faune de France. Hyménoptères Vespiformes II (Eumenidae Vespidae, Masaridae, Bethylidae, Dryinidae, Embolemidae). Office Central de Faunistique, Paris
Bernays EA (1988) Host specificity in phytophagous insects: selection pressure from generalist predators. Entomol Exp Appl 49:131–140
Bernays EA, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Berryman AA (1987) The theory and classification of outbreaks. In: Barbosa P, Schultz JC (eds) Insect outbreaks. Academic Press, London, pp 3–30
Breden F, Wade MJ (1987) An experimental study of the effect of group size on larval survivorship in the imported willow leaf beetle Plagiodera versicolor (Coleoptera: Chrysomelidae). Environ Entomol 16:1082–1086
Breden F, Wade MJ (1989) Selection within and between kin groups of the imported willow leaf beetle. Am Nat 134:35–50
Brown WJ (1956) The New World species of Chrysomela L. (Coleoptera: Chrysomelidae). Can Entomol 88:1–54
Burkot TR, Benjamin DM (1979) The biology and ecology of the cottonwood leaf beetle, Chrysomela scripta (Coleoptera: Chrysomelidae), on tissue cultured hybrid Aigeiros (Populus x euramericana) subclones in Wisconsin. Can Entomol 111:551–556
Chew FS (1980) Foodplant preferences of Pieris caterpillars (Lepidoptera). Oecologia 46: 347–353
Craig TM, Price PW, Clancy KM, Waring GL, Sacchi CF (1988) Forces preventing coevolution in the three-trophic-level system: willow, a gall-forming herbivore, and parasitoid. In: Spencer KC (ed) Chemical mediation of coevolution. Academic Press, San Diego, pp 57–80
Damman H (1987) Leaf quality and enemy avoidance by the larvae of a pyralid moth. Ecology 68:88–97
Dempster JP (1960) A quantitative study of the predators on the eggs and larvae of the broom beetle, Phytodecta olivacea Forster, using the precipitin test. J Anim Ecol 29:149–167
Denno RF, Larsson S, Olmstead KL (1990) Role of enemy-free space and plant quality in host-plant selection by willow beetles. Ecology 71: 124–137
Devantoy J (1948) Les Prédateurs et les parasites de la chrysomèle du peuplier. Feuille Nat 3:85–89
Dodge KL, Price PW, Kettunen J, Tahvanainen J (1990) Preference and performance of the leaf beetle Disonycha plurigata (Coleoptera: Chrysomelidae) in Arizona and comparisons with beetles in Finland. Environ Entomol 19: 905–910
Eickwort KR (1977) Population dynamics of a relatively rare species of milkweed beetle (Ladidomera). Ecology 58:527–538
Elliott KR, Wong HR (1966) An important predator of the aspen leaf beetle, Chrysomela crotchi Brown, in Manitoba and Saskatchewan. Bi-month Res Notes For Ser Can 22:3–4
English-Loeb GM, Brody AK, Karban R (1993) Host-plant-mediated interactions between a generalist foliavore and its tachinid parasitoid. J Anim Ecol 62:465–471
Fabre JH (1891) Souvenirs entomologiques. Delagrave, Paris
Fox CW (1993) A quantitative genetic analysis of oviposition preference and larval performance on two hosts in the bruchid beetle, Callosobruchus maculatus. Evolution 47:166–175
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Hanks LM, Denno RF (1993) Natural enemies and plant water relations influence the distribution of an armored scale insect. Ecology 74:1081–1091
Hanks LM, Paine TD, Millar JG (1993) Host species preference and larval performance in the wood boring beetle Phoracantha semipunctata F. Oecologia 95:22–29
Herting B (1973) A catalogue of parasites and predators of terrestrial arthropods Section A. Host or prey/enemy. Coleoptera to Strepsiptera. Commonwealth Institute of Biological Control, Ottawa, Canada
Holdren CE, Ehrlich PR (1982) Ecological determinants of food plant choice in the checkerspot butterfly (Euphydryas editha) in Colorado. Oecologia 52:417–423
Holtzer TO, Archer TL, Norman JM (1988) Host suitability in relation to water stress. In: Heinrichs EA (ed) Plant stress-in-sect interactions. John Wiley and Sons, New York, pp 111–137
Horton DR (1989) Performance of a willow-feeding beetle, Chrysomela knabi Brown, as affected by host species and dietary moisture. Can Entomol 121: 777–780
Hunter MD (1990) Differential susceptibility to variable plant phenology and its role in competition between two insect herbivores. Ecol Entomol 15:401–408
Hunter MD, Price PW (1992) Natural variability in plants and animals. In: Hunter MD, Ohgushi T, Price PW (eds) Effects of resource distribution on animal-plant interactions. Academic Press, San Diego, pp 1–12
Iwata K (1976) Evolution of instinct: Comparative ethology of Hymenoptera. Amerind, New Delhi
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Jolivet P (1950) Les parasites, prédateurs et phorétiques des Chrysomeloidea (Coleoptera) de la faune Franco-Belge. Bull Inst Sci Nat Belg 26:1–39
Julkunen-Tiitto R (1986) A chemotaxonomic survey of phenolics in leaves of northern Salicaceae species. Phytochemistry 25:663–667
Julkunen-Tiitto R, Tahvanainen J (1989) The effect of the sample preparation method of extractable phenolics of Salicaceae species. Planta Medica 55:55–58
Kanervo V (1946) Studies of the natural enemies of the alder leaf beetle, Melasoma aenea L. (Col., Chrysomelidae). (in Finnish with German summary). Ann Zool Soc Zool Bot Fenn 12:1–202
Kearsley MJC, Whitham TG (1989) Developmental changes in resistance to herbivory: implications for individuals and populations. Ecology 70: 422–434
Kirk RE (1982) Experimental design: procedures for the behavioral sciences. Wadsworth, Belmont, California
Krombein KV (1967) Trap nesting wasps and bees: life histories, nests, and associates. Smithsonian Press, Washington, D.C.
Lawrence WS (1990) The effects of group size and host species on development and survivorship of a gregarious caterpillar Halisidota caryae (Lepidoptera: Arctiidae). Ecol Entomol 15:53–62
Lindroth RL, Pajutee MS (1987) Chemical analysis of phenolic glycosides: art, facts, artifacts. Oecologia 74:144–148
Manning SJ (1991) The relative effects of some biotic and abiotic factors on growth and productivity of two Owens valley shrubs: Haplopappus cooperi and Chrysothamnus teretifolius. In: Hall CA, Doyle-Jones V, Widawski B (eds) Natural history of eastern California and high-altitude research, University of California (White Mountain Research Station Symposium). The Regents of the University of California, Los Angeles, pp 21–34
Mattson WJ, Scriber JM (1987) Nutritional ecology of insect folivores of woody plants: nitrogen, water, fiber, and mineral considerations. In: Slansky F, Rodriguez JG (eds) The nutritional ecology of insects, mites, and spiders. Wiley, New York, pp 105–146
McQuate GT, Connor EF (1990) Insect responses to plant water deficits. II. Effect of water deficits in soybean plants on the growth and survival of Mexican bean beetle larvae. Ecol Entomol 15:433–445
Meier B (1988) Analytik, chromatographisches Verhalten und potentielle Wirksamkeit der Inhaltsstoffe salicylathaltiger Arzneipflanzen Mitteleuropas. Habilitationsschrift. Swiss Federal Institute of Technology, Zürich
Neter J, Wasserman W, Kutner MH (1985) Applied linear statistical models: regression, analysis of variance, and experimental designs. Irwin, Homewood, Illinois
Palo RT (1984) Distribution of birch (Betula spp.), willow (Salix spp.) and poplar (Populus spp.) secondary metabolites and their potential role as chemical defense against herbivores. J Chem Ecol 10:499–520
Palokangas P, Neuvonen S (1992) Differences between species and instars of leaf beetles in the probability to be preyed on. Ann Zool Fenn 29:273–278
Pasteels JM, Braekman JC, Daloze D, Ottinger R (1982) Chemical defence in chrysomelid larvae and adults. Tetrahedron 38:1891–1897
Pasteels JM, Rowell-Rahier M, Braekman JC, Dupont A (1983) Salicin from host plant as precursor for salicylaldehyde in defensive secretion of chrysomeline larvae. Physiol Entomol 8:307–314
Pasteels JM, Rowell-Rahier M, Braekman JC, Daloze D (1984) Chemical defenses in leaf beetles and their larvae: their ecological, evolutionary and taxonomic significance. Biochem Syst Ecol 12:395–406
Pasteels JM, Rowell-Rahier M, Raupp MJ (1988) Plant-derived defense in chrysomelid beetles. In: Barbosa P, Letourneau DK (eds) Novel aspects of insect-plant interactions. Wiley, New York, pp 235–271
Penev D, Ovcharov D (1992) Study of the biology of Melasoma vigintipunctata L. (Chrysomelidae, Coleoptera) (in Bulgarian with English summary). Nauka gor (For Sci) 29:70–76
Price PW, Clancy KM (1986) Multiple effects of precipitation on Salix lasiolepis and populations of the stem-galling sawfly Euura lasiolepis. Ecol Res 1:1–14
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Rank NE (1992) Host plant preference based on salicylate chemistry in a willow leaf beetle (Chrysomela aeneicollis). Oecologia 90:95–101
Rank NE, Smiley JT (in press) Host-plant effects on Parasyrphus melanderi Curran (Diptera: Syrphidae) feeding on a willow leaf beetle Chrysomela aeneicollis Schaeffer (Coleoptera: Chrysomelidae). Ecol Entomol
Rausher MD (1980) Host abundance, juvenile survival, and oviposition preference in Battus philenor. Evolution 34:342–355
Reese JC, Beck SD (1978) Interrelationships of nutritional indices and dietary moisture in the black cutworm (Agrotis ipsilon) digestive efficiency. J Insect Physiol 24:473–479
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Richards OW (1980) Handbook for the identification of British insects: Soliodea, Vespoidea, and Sphecoidea. Royal Entomological Society of London, London
Richards OW, Waloff N (1961) A study of a natural population of Phytodecta olivacea (Forster) (Coleoptera, Chrysomelidae). Philos Trans R Soc London Ser B Biol Sci 244:205–257
Roininen H, Tahvanainen J (1989) Host selection and larval performance of two willow-feeding sawflies. Ecology 70:129–136
Rowell-Rahier M (1984) The food plant preferences of Phratora vitellinae (Coleoptera: Chrysomelidae). A: field observations. Oecologia 64:369–374
Rowell-Rahier M, Pasteels JM (1982) The significance of salicin for a Salix-feeder, Phratora (Phyllodecta) vitellinae. In: Visser JH, Minks AK (eds) Proceedings of the 5th international symposium on insect-plant relationships. Pudoc, Wageningen, pp 73–79
SAS (1988) SAS/STAT Guide for Personal Computers. Version 6.03 edition. SAS Institute Inc., Cary, North Carolina
Schneider F (1953) Syrphus nigritarsus Zett. Ein Ei- und Larvenräuber von Melasoma (Chrysom., Col.). Tijdsch Plantenziekten 59:192–194
Scriber JM (1977) Limiting effects of low leaf-water content on the nitrogen utilization, energy budget, and larval growth on Hyalophora cecropia (Lepidoptera: Saturniidae). Oecologia 28:269–287
Smereka EP (1965) The life history and habits of Chrysomela crotchi Brown (Coleoptera: Chrysomelidae) in northwestern Ontario. Can Entomol 97:541–549
Smiley JT (1978) Plant chemistry and the evolution of host specificity: new evidence from Heliconius and Passiflora. Science 201:745–747
Smiley JT, Rank NE (1986) Predator protection versus rapid growth in a montane leaf beetle. Oecologia 70:106–112
Smiley JT, Rank NE (1991) Bitterness of Salix along the north fork of Big Pine Creek, eastern California: species and community elevational trends. In: Hall CA, Doyle-Jones V, Widawski B (eds) Natural history of eastern California and high-altitude research, University of California (White Mountain Research Station Symposium). The Regents of the University of California, Los Angeles, pp 132–147
Smiley JT, Horn JH, Rank NE (1985) Ecological effects of salicin at three trophic levels: new problems from old adaptations. Science 229:649–651
Smiley KS (1991) Aggregation benefits in a willow leaf beetle along an elevation gradient. In: Hall CA, Doyle-Jones V, Widawski B (eds) Natural history of eastern California and high-altitude research, University of California. White Mountain Research Station Symposium. The Regents of the University of California, Los Angeles, pp 148–160
Strauss SY (1991) Direct, indirect, and cumulative effects of three native herbivores on a shared host plant. Ecology 72:543–558
Tahvanainen J, Julkunen-Tiitto R, Kettunen J (1985) Phenolic glycosides govern the food selection pattern of willow feeding leaf beetles. Oecologia 67:52–56
Thompson JN (1988) Evolutionary ecology of the relationship between eviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl 47:3–14
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Via S (1990) Ecological genetics and host adaptation in herbivorous insects: the experimental study of evolution in natural and agricultural systems. Annu Rev Entomol 35:421–446
Wade MJ, Breden FJ (1986) Life history of natural populations of the imported willow leaf beetle, Plagiodera versicolor (Coleoptera: Chrysomelidae) Ann Entomol Soc Am 79:73–79
Wallace JB, Blum MS (1969) Refined defensive mechanisms in Chrysomela scripta. Ann Entomol Soc Am 62:503–506
Yamane S (1990) A revision of the Japanese Eumenidae (Hymenoptera, Vespoidea). Insecta Matsum 43:1–189
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rank, N.E. Host-plant effects on larval survival of a salicin-using leaf beetle Chrysomela aeneicollis Schaeffer (Coleoptera: Chrysomelidae). Oecologia 97, 342–353 (1994). https://doi.org/10.1007/BF00317324
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317324