Abstract
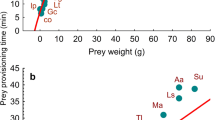
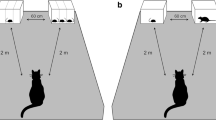
We examined factors maintaining extreme diet specialization in the snail kite (Rostrhamus sociabilis), a medium-sized hawk which feed almost exclusively on Pomacea snails, by determining why during some months kites eat crabs (Dilocarcinus dentatus) in the Ilanos of Venezuela. We offered snails and crabs of different sizes to wild free-flying birds to develop estimates for a prey choice model. Handling times of Pomacea doliodes snails averaged 90±39 s and were positively correlated with snail size. Handling times for crabs (x=353±130 s) were significantly longer and exhibited greater variation than for snails, and were not correlated with crab size. Edible crab tissues had greater dry weights and contained more energy (25.37 kJ/g) than tissues of snails (16.91 kJ/g). Total energy of crabs was much greater than that of snails, and total energy of both foods was highly related to body length. We constructed an allometric equation for profitability of snails and crabs. Snails were more profitable than all but the largest crabs, but estimates of variance in profitability were greater for crabs. Predictions from the model were tested by offering crabs that represented equal, greater and much greater profitability than snails, to determine whether kites chose prey according to profitability. Only 15.6% of 289 food items chosen were crabs. Half of the 18 kites tested did not eat crabs and only 3 birds switched from snails to more profitable crabs. Four fledglings showed no preference for snails. The role of neophobia in food choice was investigated by offering unfamiliar snails (Pomacea urceus) to kites. Kites exhibited neophobic behaviors, and 5 of 12 birds chose not to capture P. urceus. Two-thirds of the 12 snails chosen were rejected immediately, but the others were handled efficiently (x=133±89 s). Although morphological adaptations allow kites to specialize on snails, the costs of specialization were overcome for kites when the profitability of alternative food increased sufficiently. Our results suggest a role for behavioral conservatism, in the form of risk-averse foraging and neophobia, in maintaining severe diet specialization in the snail kite.
Similar content being viewed by others
References
Alves de Magalhães C (1990) Hábitos alimentares e estratégia de forrageamento de Rostrhamus sociabilis no Pantanal de Mato Grosso, Brasil. Ararajuba 1:95–98
Beissinger SR (1983) Hunting behavior, prey selection, and energetics of snail kites in Guyana: consumer choice by a specialist. Am Nat 136:20–38
Beissinger SR (1987) Anisogamy overcome: female strategies in snail kites. Am Nat 129:486–500
Beissinger SR (1988) The snail kite of North American birds, volume IV. In: Palmer RS (ed) Handbook. Yale University Press, New Haven, pp 148–165
Beissinger SR (1990a) Alternative foods of a diet specialist, the snail kite. Auk 107:327–333
Beissinger SR (1990b) Experimental brood manipulations and the monoparental threshold in snail kites. Am Nat 136:20–38
Beissinger SR, Snyder NFR (1987) Mate desertion in the snail kite. Anim Behav 35:477–487
Beissinger SR, Thomas BT, Strahl SD (1988) Vocalizations, food habits, and nesting biology of the slender-billed kite with comparisons to the snail kite. Wilson Bull 100:604–616
Benkman CW (1988) Seed-handling ability, bill structure, and the cost of specialization for cross-bills. Auk 105:715–719
Benkman CW, Lindholm AK (1991) The advantages and evolution of a morphological novelty. Nature 349:519–520
Bourne GR (1985a) Field tests of micropatch and prey-size selection by snail kites Rostrhamus sociabilis. Ibis 127:141–147
Bourne GR (1985b) The role of profitability in snail kite foraging. J Anim Ecol 54:697–709
Burky AJ (1975) Growth and biomass production of an amphibian snail, Pomacea urceus (Müller) from the Venezuelan savannah. Proc Malacalog Soc Lond 41:127–143
Burky KA, Burky AJ (1977) Buoyancy changes as related to respiratory behavior in an amphibious snail, Pomacea urceus (Müeller), from Venezuela. Nautillus 91:97–104
Caraco T (1981) Energy budgets, risk, and foraging in dark-eyed juncos (Junco hyemalis). Behav Ecol Sociobiol 8:213–217
Caraco T, Martindale S, Whittam TS (1980) An empirical demonstration of risk-sensitive foraging preferences. Anim Behav 28:820–830
Cartar RV (1991) A test of risk-sensitive foraging in wild bumble bees. Ecology 72:888–895
Coppinger RP (1969) The effect of experience and novelty on avian feeding behavior with reference to the evaluation of warning coloration on butterflies. I. Reactions of wild-caught adult blue jays to novel insects. Behaviour 35:45–60
Coppinger RP (1970) The effect of experience and novelty on avian feeding with reference to the warning coloration of butterflies. II. Reactions of naive birds to novel insects. Am Nat 104:323–337
Donnay TJ, Beissinger SR (1993) Apple snail (Pomacea doliodes) and freshwater crab (Dilocarcinus dentatus) population fluctuations in the Ilanos of Venezuela. Biotropica 25:206–214
Edwards TC Jr (1989) The ontogeny of diet selection in fledging ospreys. Ecology 70:881–896
Eisenberg JF (ed) (1979) Vertebrate ecology in the northern neotropics. Smithsonian Institution Press, Washington, DC
Ezekiel M, Fox KA (1963) Methods of correlation and regression analysis. Wiley New York
Forbes LS (1989) Prey defenses and predator handling behaviour: the dangerous prey hypothesis. Oikos 55:155–158
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Greenberg R (1983) The role of neophobia in determining the degree of foraging specialization in some migrant warblers. Am Nat 122:444–453
Greenberg R (1985) The role of neophobia in foraging site selection of a tropical migrant bird: an experimental study. Proc Natl Acad Sci USA 81:3778–3780
Greenberg R (1987) Development of dead leaf foraging in a tropical migrant warbler. Ecology 68:130–141
Guilford T, Dawkins MS (1987) Search images not proven; a reappraisal of recent evidence. Anim Behav 35:1838–1845
Haverschmidt F (1962) Notes on the feeding habits and food of some hawks of Surinam. Condor 64:154–158
Haverschmidt F (1970) Notes on the snail kite in Surinam. Auk 87:580–584
Howell AH (1932) Florida bird life. Coward-McCann, New York
Keating JR, Robel RJ, Adams AW, Behnke KC, Kemp KE (1992) Role of handling time in selection of extruded food morsels by two granivorous bird species. Auk 109:863–868
Klopfer PH (1967) Behavioral aspects of habitat selection: a preliminary report on stereotypy in foliage preferences of birds. Wilson Bull 77:376–381
Krebs JR (1980) Optimal foraging, predation risk and territory defence. Ardea 68:83–90
Krebs JR (1991) The case of the curious bill. Nature 349:465
Labinger Z, Katir G, Benjamin Y (1991) Prey size choice by captive pied kingfishers, Ceryle rudis L. Anim Behav 42:969–975
Levins R (1968) Evolution in changing environments. Princeton University Press, Princeton, NJ
Lotem A, Schechtman E, Katir G (1991) Capture of submerged prey by little egrets, Egretta garzetta garzetta: strike depth, strike angle, and the problem of light refraction. Anim Behav 42:341–346
MacArthur RH (1972) Geographical ecology. Harper and Row, New York
Mader WJ (1981) Notes on nesting raptors in the Ilanos of Venezuela. Condor 83:48–51
Martin AC, Zim HS, Nelson AL (1951) American wildlife and plants: a guide to wild food habits. Dover, New York
Martinez del Rio C, Stevens BR (1988) Physiological constraint on feeding behavior: intestinal membrane disaccharidases of the starling. Science 243:794–796
Meyer A (1989) Cost of morphological specialization: feeding performance of the two morphs in trophically polymorphic cichlid fish, Ciclasoma citrinellum. Oecologia 80:431–436
O'Connell M (1989) Population dynamics of Neotropical small mammals in seasonal habitats. J Mammal 70:532–548
Partridge L, Green P (1987) An advantage for specialist feeding in jackdaws, Corvus monedula. Anim Behav 35:982–990
Price T (1987) Diet variation in a population of Darwin's finches. Ecology 68:1015–1028
Pyke GH (1984) Optimal foraging theory: a critical review. Annu Rev Ecol Syst 15:523–575
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
Real L (1991) Animal choice behavior and the evolution of cognitive architecture. Science 253:980–986
Real L, Caraco T (1986) Risk and foraging in stochastic environments. Annu Rev Ecol Syst 17:371–390
Richards LJ (1983) Hunger and the optimal diet. Am Nat 122:326–334
Schoener TW (1969a) Optimal size and specialization in constant and fluctuating environments: an energy-time approach. In: Diversity and stability in ecological systems (Brookhaven Symposia in Biology no. 22):103–114
Schoener TW (1969b) Models of optimal size for solitary predators. Am Nat 103:277–313
Schoener TW (1971) Theory of feeding strategies. Annu Rev Ecol Syst 2:369–404
Schoener TW (1979) Generality of the size-distance relation in models of optimal feeding. Am Nat 114:902–914
Sih A (1980) Optimal behavior: can foragers balance two conflicting demands? Science 210:1041–1043
Snyder NFR, Kale HW II (1983) Mollusk predation by snail kites in Colombia. Auk 100:93–97
Snyder NFR, Snyder HA (1969) A comparative study of mollusc predation by limpkins, Everglade kites, and boat-tailed grackles. Living Bird 8:177–223
Snyder NFR, Snyder HA (1970) Feeding territories in the Everglade kite. Condor 72:492–493
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton, NJ
Stephens DW, Lynch JF, Sorenson AE, Gordon C (1986) Preference and profitability: theory and experiment. Am Nat 127:533–553
Strong DR, Lawton JH, Southwood R (1984) Insects on plants: community patterns and mechanisms. Harvard University Press, Cambridge, Mass
Sutherland WJ (1987) Why do animals specialize? Nature 325:483–484
Sutherland WJ, Anderson CW (1987) Six ways in which a foraging predator may encounter options with different variances. Biol J Linn Soc 30:99–114
Sykes PW Jr, Kale HW II (1974) Everglades Kites feed on nonsnail prey. Auk 91:818–820
Tinbergen L (1960) The natural control of insects in pine woods. I. Factors influencing the intensity of predation by songbirds. Arch Neer Zool 13:265–343
Tuttle EM, Wulfson L, Caraco T (1990) Risk-aversion, relative abundance of resources and foraging preference. Behav Ecol Sociobiol 26:165–171
Voous KH, van Dijk T (1973) How do snail kites extract snails from their shells? Ardea 61:179–185
Waddington KD, Holden LR (1979) Optimal foraging: on flower selection by bees. Am Nat 114:179–196
Weller MW (1967) Notes on some marsh birds of Cape San Antonio, Argentina. Ibis 109:391–411
Werner TK, Sherry TW (1987) Behavioral feeding specialization in Pinaroloxias inornata, the “Darwin's finch” of Cocos Island, Costa Rica. Proc Natl Acad Sci USA 84:5506–5510
Wilkinson L (1990) SYSTAT: the system for statistics. Systat Inc., Evanston, Ill
Woodin MC, Woodin CD (1981) Everglade kite predation on a soft-shelled turtle. Fla Field Nat 9:64
Wunderle JM Jr (1991) Age-specific foraging proficiency in birds. Curr Ornithol 8:273–324
Wunderle JM Jr, Cotto-Navarro Z (1988) Constant versus variable risk-aversion in foraging bannaquits. Ecology 69:1434–1438
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beissinger, S.R., Donnay, T.J. & Walton, R. Experimental analysis of diet specialization in the snail kite: the role of behavioral conservatism. Oecologia 100, 54–65 (1994). https://doi.org/10.1007/BF00317130
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317130