Summary
The pharmacokinetics of ximoprofen, a potent new non-steroidal anti-inflammatory agent, has been investigated in normal healthy subjects and in patients with hepatic or renal disease.
After intravenous infusion of 22.8 mg to healthy subjects, plasma ximoprofen concentrations declined in a polyexponential manner with a terminal phase half-life of 1.9 h. The systemic clearance of ximoprofen was 115 ml·min−1 and the volumes of distribution were 18.0 l Vz and 13.8 l Vss. Ximoprofen was 80–90% bound to plasma proteins. The systemic availabilities (f) of orally and rectally administered doses of 30 mg of ximoprofen were 98% and 56% respectively and, in the case of the rectal dose, absorption appeared to be prolonged leading to “flip-flop” kinetics.
After single oral doses of 30 mg of ximoprofen to patients with hepatic disease, half-life (2.2 h), peak plasma concentrations (1.55 μg·ml−1 cf 1.04 μg·ml−1 in healthy subjects) and areas under the curve (6.12 μg·h·ml−1 cf 3.54 μg·h·ml−1 in healthy subjects) were significantly different from those in healthy subjects.
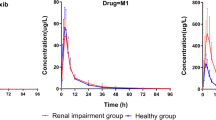
After single oral doses of 30 mg of ximoprofen to patients with renal disease, pharmacokinetic parameters of half-life (4.0 h), mean residence time (6.0 h) and area under the curve (9.2 μg·h·ml−1) were significantly different from those in healthy subjects. There were no significant differences in pharmacokinetic parameters between patients having differing degrees of renal disease.
These data nevertheless suggest that accumulation of ximoprofen in hepatic or renal disease would be of slight or negligible clinical relevance and that no alteration of the dose regimen (up to 15 mg twice daily) may be required when ximoprofen is administered in these disease states.
Similar content being viewed by others
References
Adams SS (1987) Non-steroidal anti-inflammatory drugs, plasma half-lives and adverse reactions. Lancet II: 1204–1205
Bass NM, Williams RL (1988) Guide to drug dosage in hepatic disease. Clin Pharmacokinet 15: 396–420
Bennett WM (1988) Guide to drug dosage in renal failure. Clin Pharmacokinet 15: 326–354
Caporal R, Cottin S, Dreyfus P, Guidet M, Jauffret P, Schoen E, Laffez B (1987) Ximoprofen and inflammatory rheumatoid disease. Clin Exp Rheumatol 5 [Suppl 2] Abstr P 405
Chiou WL (1978) Critical evaluation of the potential error in pharmacokinetic studies of using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve. J Pharmacokinet Biopharm 6: 539–546
Cutler DJ (1979) A linear recirculation model for drug disposition. J Pharmacokinet Biopharm 7: 101–116
Davies O (1961) Linear relationships between two variables. Statistical Methods in Research and Production, 3rd Ed, Oliver and Boyd, London, pp 150–207
Flower RJ, Moncada S, Vane JR (1985) Analgesic antipyretics and anti-inflammatory agents; drugs employed in the treatment of gout. In: Gilman AG, Gilman LS, Rall T, Murad F (eds) The Pharmacological Basis of Therapeutics. 7th edn, Macmillan, New York, pp 674–715
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd Edn. Dekker, New York
Langenbucher F (1982) Numerical convolution/deconvolution as a tool for correlating in vitro with in vivo drug availability. Pharm Ind 44: 1166–1171
Lin JH, Cocchetto DM, Duggan DE (1987) Protein binding as a primary determinant of the clinical pharmacokinetic properties of non-steroidal anti-inflammatory drugs. Clin Pharmacokinet 12: 402–432
Maillard J, Langlois M, Delaunay P, VoVan T, Meingan JP, Rapin M, Morin R, Manuel C, Mazmanian C (1977) Anti-inflammatoires derives de l'acide phenylacetique. 111. Derives oxygenes de l'acide cyclohexyl-4 phenylacetique. Eur J Med Chem Chim Ther 12: 161–171
Mayo BC, Chasseaud LF, Hawkins DR, Taylor IW, Legeai J (1990) The metabolic fate of 14C-ximoprofen in rats, baboons and humans. Xenobiotica 20: 233–246
Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60: 646–649
Riegelman S, Collier P (1980) The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm 8: 509–534
Slater JDH (1969) In: Clinical Physiology, Campbell EJM, Dickinson CJ, Slater JDH (eds) Blackwell, Oxford
Taylor IW, Chasseaud LF (1989) Determination of ximoprofen in human plasma by gas chromatography. J Chromatogr 495: 275–280
Wagner JG (1983) Significance of ratios of different volumes of distribution in pharmacokinetics. Biopharm Drug Dispos 4: 263–270
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, I.W., Taylor, T., James, I. et al. Pharmacokinetics of the anti-inflammatory drug ximoprofen in healthy subjects and in disease states. Eur J Clin Pharmacol 40, 101–106 (1991). https://doi.org/10.1007/BF00315147
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315147