Abstract
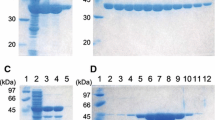
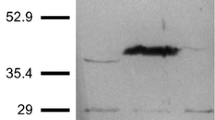
The α-acetolactate synthase from Leuconostoc mesenteroides subsp. cremoris was purified to homogeneity in SDS-PAGE. The enzyme is a trimer of 3×55,000 Da. It was unstable but could be preserved by addition of pyruvate and thiamine pyrophosphate in the buffer. The enzyme exhibits Michaelis-Menten kinetics, and K m for pyruvate is 10 mM. Three intermediates in glucose metabolism (ATP, 3-phosphoglycerate, and phosphoenolpyruvate) exhibit a noncompetitive inhibition towards the enzyme. This enzyme does not require any divalent metal ion for activity. The α-acetolactate synthase from Leuconostoc mesenteroides subsp. cremoris is not inhibited by the branched-chain amino acids (valine, leucine, and isoleucine), is FAD independent, and displays an optimal activity at pH 5.3. Therefore, it can be concluded that the purified enzyme belongs to the catabolic α-acetolactate synthases, involved in the 2,3-butanediol pathway but not in branchedchain amino acids biosynthesis.
Similar content being viewed by others
Literature Cited
Basso AL, Ricca E, Caruso C, Ferrara L, De Felice M (1993) Acetohydroxy acid synthase and threonine deaminase activities, and the biosynthesis of isoleucine-leucine-valine in Streptococcus bovis. Res Microbiol 144:539–545
Cogan TM, O'Dowd M, Mellerick D (1981) Effect of pH and sugar on acetoin production from citrate by Leuconostoc lactis. Appl Environ Microbiol 41:1–8
Cogan TM, Fitzgerald J, Doonan S (1984) Acetolactate synthase of leuconostoc lactis and its regulation of acetoin production. J Dairy Res 51:597–604
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of Lactobacill. J Appl Bacteriol 23:130–135
Friden P, Donegan J, Mullen J, Tsui P, Freundlich M, Eoyang L, Weber R, Silverman PM (1985) The ilvB locus of Escherichia coli K12 is an operon encoding both subunits of acetohydroxy acid synthase I. Nucleic Acids Res 13:3979–3993
Godon JJ, Chopin MC, Ehrlich SD (1992) Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol 174:6580–6589
Huseby NE, Christensen TB, Olse BR, Stormer F (1971) The pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. Subunit structure. J Biochem 20:209–214
Juni E (1952) Mechanisms of formation of acetoin by bacteria. J Biol Chem 195:715–726
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosenborough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193:265–275
Malthe-Sorenssen D, Stormer FC (1970) The pH6 acetolactateforming enzyme from Serratia marcescens. Purification and properties. Eur J Biochem 14:127–132
Monnet C, Phalip V, Schmitt P, Diviès C (1994a) Comparison of the α-acetolactate synthase and α-acetolactate decarboxylase in Lactococcus and Leuconostoc spp. Biotechnol Lett 16:257–262
Monnet C, Schmitt P, Diviès C (1994b) Diacetyl production in milk by an α-acetolactic accumulating strain of Lactococcus lactis spp. lactis biovar. diacetylactis. J Dairy Sci 77:2916–2924
Nuraida L, Owens JD (1992) Acetoin production in Leuconostoc mesenteroides NCDO 518. FEMS Microbiol Lett 91:245–250
Peng HL, Wang PY, Wu CM, Hwang DC, Chang HY (1992) Cloning, sequencing and heterologous expression of a Klebsiella pneumoniae gene encoding an FAD-independent acetolactate synthase. Gene 117:125–130
Schmitt P, Diviès C (1992) Effects of varying citrate levels on C4 compounds formation and on enzyme levels in Leuconostoc mesenteroides subsp. cremoris grown in continuous culture. Appl Microbiol Biotechnol 37:426–430
Snoep JL, Teixeira De Mattos MJ, Starrenburg MJC, Hugenholtz J (1992) Isolation, characterization and physiological role of the pyruvate dehydrogenase complex and α-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol 174:4838–4841
Squires CH, De Felice M, Devereux J, Calvo JM (1983) Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res 11:5299–5313
Starrenburg MJC, Hugenholtz J (1991) Citrate fermentation by Lactococcus and Leuconostoc spp. Appl Environ Microbiol 57:3535–3540
Stormer FC (1975) 2,3-Butanediol biosynthetic system in Aerobacter aerogenes. Methods Enzymol 41:518–533
Thomas TD, Ellwood DC, Longyear C (1979) Change from homo-to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostats cultures. J Bacteriol 138:109–117
Verhue JD, Tjan FSB (1991) Study of the citrate metabolism of Lactococcus lactis subsp. lactis biovar. diacetylactis by means of 13C nuclear magnetic resonance. Appl Environ Microbiol 57:3371–3377
Weinstock O, Sella C, Chipman DM, Barak Z (1992) Properties of subcloned subunits of bacterial acetohydroxyacid synthases. J Bacteriol 147:5560–5566
Wek RC, Hauser CA, Hatfield GW (1985) The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozyme. Nucleic Acids Res 13:3995–4010
Westerfeld WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161:495–502
Yang JH, Kim SS (1993) Purification and characterization of the valine sensitive acetolactate synthase from Serratia marcescens ATCC 25419. Biochim Biophys Acta 1157:178–184
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phalip, V., Schmitt, P. & Diviès, C. Purification and characterization of the catabolic α-acetolactate synthase from Leuconostoc mesenteroides subsp. cremoris . Current Microbiology 31, 316–321 (1995). https://doi.org/10.1007/BF00314587
Issue Date:
DOI: https://doi.org/10.1007/BF00314587