Abstract
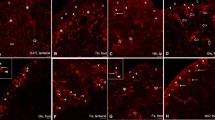
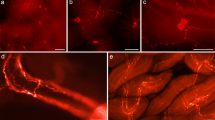
The distribution and characterization of dopamine-containing neurons are described in the different ganglia of the central nervous system of Helix on the basis of the distribution of tyrosine hydroxylase immunoreactive (TH-ir) and dopamine immunoreactive (DA-ir) neurons. Both TH-ir and DA-ir cell bodies of small diameter (10–25 μm) can be observed in the buccal, cerebral and pedal ganglia, dominantly on their ventral surface, and concentrated in small groups close to the origin of the peripheral nerves. The viscero-parietal-pleural ganglion complex is free of immunoreactive cell bodies but contains a dense fiber system. The largest number of TH-ir and DA-ir neurons can be detected in the pedal, and cerebral ganglia. The average number of TH-ir and DA-ir neurons significantly differs but all the identifiable groups of TH-ir neurons also show DA-immunoreactivity. Therefore, we consider the TH-ir neurons in those groups as being DA-containing neurons. The amounts of DA in the different ganglia assayed by high performance liquid chromatography correspond to the distribution and number of TH-ir and DA-ir neurons in the different ganglia. The axon processes of the labeled small-diameter neurons send thin proximal branches toward the cell body layer but only rarely surround cell bodics, whereas distally they give off numerous branches in the neuropil and then leave the ganglion through the peripheral nerves. In the cerebral ganglia, the analysis of the TH-ir pathways indicates that the largest groups of labeled neurons send their processes through the peripheral nerves in a topographic order. These results furnish morphological evidence that DA-containing neurons of Helix pomatia have both central and peripheral roles in neuronal regulation.
Similar content being viewed by others
References
Audesirk GJ (1985) Amine-containing neurones in the brain of Lymnaea stagnalis: distribution and effects of precursors. Comp Biochem Physiol [A] 81:359–365
Berry MS, Cottrell GA (1975) Excitatory, inhibitory and bi-phasic synaptic potentials mediated by an identified dopamine-containing neuron. J Physiol (Lond) 244:589–612
Berry MS, Cottrell GS, Pentreath VW, Powel B (1974) Catecholamine microdialysis and morphology of giant dopaminecontaining neurone. J Physiol (Lond) 237:19–20
Bokisch AJ, Walker RJ (1986) The ionic mechanism associated with the action of putative transmitters on identified neurons of the snail, Helix aspersa. Comp Biochem Physiol [C] 84:231–241
Bunney BS, Chiodo LA, Grace AA (1991) Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse 9:79–94
Carpenter D, Breese G, Schanberg S, Kopin I (1971) Serotonin and dopamine: distribution and accumulation in Aplysia nervous and non-nervous tissues. Int J Neurosci 2:49–56
Casey C, Winlow W (1985) Cellular localization of serotonin by glyoxylic acid condensation in Lymnaea. J Physiol (London) 369:169
Cottrell GA (1977) Identified amine-containing neurones and their synaptic connexions. Neuroscience 2:1–18
Cottrell GA, Abernethy KB, Barrand MA (1979) Large amine-containing neurons in the central ganglia of Lymnaea stagnalis. Neuroscience 4:685–689
Croll RP, Chiasson BJ (1990) Distribution of catecholamines and of immunoreactivity to substances like vertebrate enzymes for the synthesis of cathecholamines within the central nervous system of the snail, Lymnaea stagnalis. Brain Res 525:101–114
Dahl E, Falk B, Lindquist M, Mecklenburg C von (1962) Monoamines in molluscan neurons. K Fysiogr Saellsk Lund Faerh 32:89–92
Dahl E, Falk B, Mecklenburg C von, Myhrberg H (1966) Neuronal localisation of dopamine and 5-hydroxytryptamine in some molluscs. Z Zellforsch 71:489–498
Elekes K (1991) Serotonin-immunoreactive varicosities in the cell body region and neural sheath of the snail, Helix pomatia ganglia: an electron microscopic immunocytochemical study. Neuroscience 42:583–591
Elekes K, Kemenes G, Hiripi L, Geffard M, Benjamin PR (1991) Dopamine-immunoreactive neurones in the central nervous system of the pond snail Lymnaea stagnalis. J Comp Neurol 307:214–224
Geffard M, Buijs RM, Seguela P, Pool WC, LeMoal M (1984a) First demonstration of highly specific and sensitive antibodies against dopamine. Brain Res 294:161–165
Geffard M, Kah O, Onteniente B, Seguela P, LeMoal M, Delage M (1984b) Antibodies to dopamine: radioimmunological study of specificity in relation to immunocytochemistry. J Neurochem 42:1593–1599
Goldstein R, Kistler HB Jr, Steinbusch HWM, Schwartz JH (1984) Distribution of serotonin-immunoreactivity in the juvenile Aplysia. Neuroscience 11:535–547
Gospe SM (1983) Mini review: studies of dopamine pharmacology in molluscs. Life Sci 33:1945–1957
Haydon PG, Winlow W (1981) Morphology of the giant dopamine containing neuron, R.Pe.D1, in Lymnaea stagnalis revealed by Lucifer yellow CH. J Exp Biol 94:149–157
Hernádi L (1992) Somatotopic representation of the head areas in the cerebral ganglion of the snail Helix pomatia. Acta Biol Hung 43:221–230
Hernádi L, Elekes K,(1993) Peptidergic and aminergic centers in the Helix cerebral ganglia: somatotopy and immunocytochemistry. Acta Biol Hung 44:89–92
Hernádi L, Elekes K, S-Rózsa K (1989) Distribution of serotonin containing neurons in the central nervous system of the snail Helix pomaria. Comparison of immunocytochemical and 5,6-dihydroxytryptamine-labelling. Cell Tissue Res 25:313–323
Hernádi L, Hiripi L, Vehovszky Á, Kemenes G, S-Rózsa K (1992a) Ultrastructural, biochemical and electrophysiological changes induced by 5,6-dihydroxytryptamine in the CNS of the snail Helix pomatia L. Brain Res 578:221–234
Hernádi L, S-Rózsa K, Jahan-Parwar B, Carpenter DO (1992b) A topography and ultrastructural characterization of in vivo 5,7-dihydroxytryptamine-labeled serotonin-containing neurons in the central nervous system of Aplysia californica. Cell Mol Neurobiol 12:317–326
Kabotyanski EA, Sakharov DA (1988) Monoamine-dependent behavioral states in the pteropod mollusc Clione limacina. Symp Biol Hung 36:463–477
Kemenes G, Elekes K, Hiripi L, Benjamin PR (1989) A comparison of four techniques for mapping the distribution of serotonin and serotonin-containing neurons in fixed and living ganglia of the snail, Lymnaea. J Neurocytol 18:193–208
Kemenes G, Hiripi L, Benjamin PR (1990) Behavioral and biochemical changes in the feeding system of Lymnaea induced by dopamine and serotonin neurotoxins 6-hydroxydopamine and 5,6-dihydroxytryptamine. Philos Trans R Soc Lond [Biol] 329:243–255
Kerkut GA, Sedden CB, Walker RJ (1966) The effect of DOPA, α-methyl-DOPA and reserpine on the dopamine content of the brain of the snail, Helix aspersa. Comp Biochem Physiol 18:921–930
Kiriakides MA, McCrohan CR (1989) Effect of putative neuromodulators on rhythmic buccal motor output in Lymnaea stagnalis. J Neurobiol 20:635–650
Leake LD, Walker RJ (1980) Invertebrate pharmacology. Blackie, Glasgow
Longley RD, Longley AJ (1986) Serotonin immunoreactivity of neurons in the gastropod Aplysia californica. J Neurobiol 17:339–358
Marsden C, Kerkut GA (1970) The occurrence of monoamines in Planorbis corneus. A fluorescence microscopic and microspectrophotometric study. Comp Gen Pharmacol 1:101–116
McCaman MW, Ono JK, McCaman RE (1979) Dopamine measurements in molluscan ganglia and neurons using a new sensitive assay. J Neurochem 32:1111–1113
Nässel DR, Elekes K (1984) Ultrastructural demonstration of serotonin-immunoreactivity in the nervous system of an insect (Calliphora erythrocephala). Neurosci Lett 48:203–210
Nässel DR, Elekes K (1992) Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res 267:147–167
Neckameyer WS, Quinn WG (1989) Isolation and characterization of the gene for Drosophila tyrosine hydroxylase. Neuron 2:1167–1175
Osborne NN (1984) Phenylethanolamine-N-methyltransferase and dopamine-β-hydroxylase immunoreactivity and occurrence of noradrenaline and adrenaline in the nervous system of the snail Helix aspersa. Cell Tissue Res 237:605–608
Powell B, Cottrell GA (1974) Dopamine in an identified neurone of Planorbis corneus. J Neurochem 22:605–606
Ruben P, Lukowiak K (1983) Modulation of the Aplysia gill withdrawal reflex by dopamine. J Neurobiol 14:271–284
S-Rózsa K (1984) The pharmacology of molluscan neurons. Prog Neurobiol 23:79–150
S-Rózsa K, Hernádi L, Kemenes G (1986) Selective in vivo labelling of serotonergic neurons by 5,6-dihydroxytryptamine in the snail Helix pomatia L. Comp Biochem Physiol [C] 85:419–425
Sakharov DA, Zs-Nagy I (1968) Localization of biogenic monoamines in cerebral ganglia of Lymnaea stagnalis L. Acta Biol Hung 19:145–157
Schulze H, Speckmann EJ,Kuhlmann D, Caspers H (1975) Topography and bioelectrical properties of identifiable neurons in the buccal ganglion of Helix pomatia. Neurosci Lett 1:277–281
Sedden CB, Walker RJ, Kerkut GA (1968) The localization of dopamine and 5-hydroxytryptamine in neurones of Helix aspersa. Symp Zool Soc (Lond) 22:19–32
Sternberger L (1979) Immunocytochemistry. Wiley, Chichester
Straub H, Kuhlmann D (1984) Identification and quantitative measurements of biogenic monoamines in the central nervous tissue of some gastropods. Comp Biochem Physiol [C] 78:319–323
Sweeney D(1963) Dopamine: its occurrence in molluscan ganglia. Science 139:1051
Syed NI, Winlow W (1991) Respiratory behavior in the pond snail Lymnaea stagnalis. II. Neural elements of the central pattern generator (CPG). J Comp Physiol [A] 169:557–568
Trimble DR, Barker DL (1984) Activation by dopamine of patterned motor output from the buccal ganglia of Helisoma trivolis. J Neurobiol 15:37–48
Tritt SH, Lowe IP, Byrne JH (1983) A modification of the glyoxylic acid induced histofluorescence technique for demonstration of catecholamines and serotonin in tissues of Aplysia californica. Brain Res 259:159–162
Vehovszky Á, Hernádi L, Elekes K, Balaban P (1993) Serotonergic input on identified command neurons in Helix. Acta Biol Hung 44:97–101
Walken RJ (1986) Transmitters and modulators. In: Willows AOD (ed) The Mollusca. Neurobiology and behaviour. Academic Press, New York, pp 279–484
Walker RJ, Holden-Dye LM, Vehovszky Á, Bokisch AJ, Cox RTL (1988) Some aspects of molluscan neuropharmacology. Symp Biol Hung 36:63–76
Wieland SJ, Gelperin A (1983) Dopamine elicits feeding motor program in Limax maximus. J Neurosci 3:1735–1745
Winlow W, Haydon PG, Benjamin PR (1981) Multiple postsynaptic actions of the giant dopamine-containing neurone R.Pe.D1 of Lymnaea stagnalis. J Exp Biol 94:137–148
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hernádi, L., Juhos, S. & Elekes, K. Distribution of tyrosine-hydroxylase-immunoreactive and dopamine-immunoreactive neurons in the central nervous system of the snail Helix pomatia . Cell Tissue Res 274, 503–513 (1993). https://doi.org/10.1007/BF00314547
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00314547