Abstract
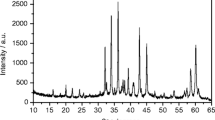
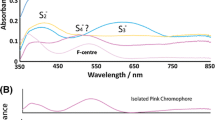
Hydrothermal scheelite was synthesized using Na2WO4 · 2 H2O mixed with CaCl2 · H2O, CaSO4 · 2 H2O or CaF2 at different temperatures (270–720° C) and 108 Pa. The morphology of the crystals depends on the starting products. The observed faces include the {112}, {114}, {011}, and {013} forms. Pure or REE doped scheelites were studied by thermoluminescence (TL), fluorescence and electron paramagnetic resonance (EPR). The main TL peaks are located near 88, 149, 216, 277, and 315 K. Results obtained with EPR or optical fluorescence have been correlated with TL measurements and show that the trivalent lanthanide elements substitute for calcium ions without site distortion. The differences in TL observed between Eu and the other doping elements are related to the greater stability of Eu2+ caused by X-irradiation.
Similar content being viewed by others
References
Anikin IN (1956) Hydrothermal synthesis of scheelite. Kristallografiya 2:195–7
Anikin IN (1957) Artificial production of scheelite crystals. Zap Vses Mineral Ova 86:459–68
Anikin IN (1959) Conditions for the hydrothermal synthesis of scheelite crystals. Zap Vses Mineral Ova 87:196–7
Bayer G (1978) Zur Kristallchemie der Zirkons und der Scheelites. Schweiz Mineral Petrogr Mitt 58:111–126
Born G, Grasser RJ, Scharmann A (1968) EPR and thermoluminescent measurements on CaWO4 crystals. Phys Status Solidi 28:583–8
Born G, Hofstaetter A, Scharmann A, Schwarz G (1970) Luminescence mechanism of tungstate phosphors. J Lumin 1–2:641–650
Caruba R, Baumer A (1980) Méthode d'obtention de minéraux de tungstène (Huebnerite, ferberite et scheelite). Soc Géol de France, 8ème R.A.S.T. Marseille p. 83
Cottrant JF (1981) Cristallographie et géochimie des terres rares dans la scheelite. Application `a quelques gisements français. Thèse 3ème Cycle, Paris
Dieke GH (1968) Spectra and Energy levels of rare earth ions in crystals. Interscience, New York
Donnay JDH, Harker D (1937) A new law of crystal morphology extending the law of Bravais. Am Mineral 22:446–467
Forrester PA, Hempstead CF (1962) Paramagnetic resonance of Tb3+ ions in CaWO4 and CaF2. Phys Rev 126:923–930
Foster RP (1977) Solubility of scheelite in hydrothermal chloride solutions. Chem Geol 20:27–43
Furtak SP, Pashkovskii MV (1969) The investigation of trapping levels in CdWO4, ZnWO4, and CaWO4. Phys Status Solidi 33:555–561
Grasser R, Scharmann A (1976) Luminescent sites in CaWO4 and CaWO4:Pb crystals. J Lumin 12–13:473–478
Hempstead CP, Bowers DK (1960) Paramagnetic resonance of impurities in CaWO4. Two states ions. Phys Rev 118:1, 131–134
Hofstaetter A, Planz J, Scharmann A (1977) Identification of thermoluminescence traps in CaWO4 and BaWO4 by EPR Measurements. Z Naturforsch 32A:957–963
Iacconi P (1979) Étude et interprétation des propriétés thermoluminescentes des zircons ZrSiO4 et Zr(SiO4)1−x(OH)4x dopés par des éléments trivalents de la série des lanthanides. Thèse, Université de Nice
Iacconi P, Caruba R (1980) Trapping Emission Centres in X-Irradiated Zircon. III Influence of trivalent rare earth impurities. Phys Status Solidi (a), 62:589–596
Mariano AN, Ring PJ (1975) Europium activated cathodoluminescence in minerals. Geochim Cosmochim Acta 39:649–660
Nasau K, Broyer AM (1962) Calcium tungstate: Czochralski growth, perfection and substitution. J Appl Phys 33:3064–3073
Peterson RG, Powell RC (1978) Energy transfer in rare-earth doped CaWO4 after edge excitation. J Lumin 16:285–296
Sayer M, Souder AD (1968) On the origin of defect states in calcium tungstate. Can J Phys 47:463–471
Treadaway MJ, Powell RC (1975) Energy transfer in samariumdoped calcium tungstate crystals. Phys Rev B 11:2, 862–874
Van Uitert LG, Soden RR (1960) Emission spectra of trivalent terbium. J Chem Phys 32:1161–1164
Zemlicka J, Barta C (1966) Scheelite single crystals growth by the Verneuil method. Krist Tech 1:281–3
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caruba, R., Iacconi, P., Cottrant, J.F. et al. Thermoluminescence, fluorescence and electron paramagnetic resonance properties of synthetic hydrothermal scheelites. Phys Chem Minerals 9, 223–228 (1983). https://doi.org/10.1007/BF00311959
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00311959