Abstract
The E∥c and E ⊥ c polarized optical absorption spectra of a variety of blue/green tourmalines and a schorl were measured from room temperature down to helium temperatures. Heat treatments at 750–800° C in air and hydrogen were carried out on several green tourmalines.
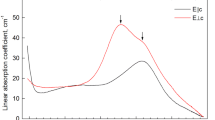
From the results obtained, absorptions at 7,900 and 13,800 cm−1 in the E∥c spectra of tourmalines are assigned to Fe2+ in the b-site. In the same polarization, bands detected at 9,000 and 13,400 cm−1 are attributed to Fe2+ in the smaller c position. In contrast to previous interpretations, the E ⊥ c polarized bands at 9,000 and 13,800 cm−1 are not assigned to single ion transitions, but are largely associated with nearest neighbour Fe2+-Fe3+ pairs. Correlations between near-infrared band absorption coefficients and FeO concentration reinforce these assignments.
The temperature dependence and the reaction to heat treatment of the strongly polarized (E⊥c≫E∥c) band near 18,000 cm−1 in blue and green tourmaline spectra are shown to be consistent with previous assignments of the band to Fe2++Fe3+→Fe3++Fe2+ charge transfer.
Similar results are discussed for broad absorptions (also E⊥c≫E∥c) found in the 22,000–25,000 cm−1 region of the spectra of certain green and brown tourmalines. It is concluded that these absorptions are due to Fe2++Ti4+→Fe3++Ti3+ charge transfer.
The proposal is made that the initial effect of heating green tourmalines in air and hydrogen is to reduce Fe3+ cations located in both b- and c-sites. Further heat treatment in air and hydrogen results in the oxidation of Fe2+→Fe3+ and leads to the generation of bands near 19,100 and 21,600 cm−1. The newly formed bands are assigned to Fe3+-Fe3+ pairs.
Similar content being viewed by others
References
Bakhtin, A.I., Minko, O. Ye, Vinokurov, V.M.: Isomorphism and colour of tourmaline. Izv. Akad. Nauk SSSR., Ser. Geol. No. 6, 73–83 (1975)
Belov, A.F., Korneev, E.V., Khimich, T.A., Belov, V.F., Korovushkin, V.V., Zheludev, I.S., Kolesnikov, I.M.: The electronic-nuclear interactions and non-equivalent positions of the iron ions in tourmaline. Zh. fiz. Khim. 49, 1683–1688 (1975) [Russ. J. Phys. Chem. 49(7), 992–996 (1975)]
Belov, V.F., Korovushkin, V.V., Belov, A.F., Korneev, E.V., Zheludev, I.S.: Nonequivalent positions of the iron ions and electron-nuclear interactions in tourmaline. Fiz. Tverd. Tela. 16, 2410–2411 (1974) [Sov. Phys. Solid State 16(8), 1568 (1975)]
Bershov, L.V., Martirosyan, V.O., Marfunin, A.S., Platonov, A.N., Tarashchan, A.N.: Colour centres in lithium tourmaline (elbaite). Krystallografiya 13, 730–732 (1967). [Sov. Phys. Cryst. 13, 629–630 (1969)]
Buerger, M.J., Burnham, C.W., Peacor, D.R.: Assessment of the several structures proposed for tourmaline. Acta. Cryst. 15, 583–590 (1962)
Burns, R.G.: Mineralogical Applications of Crystal Field Theory. Cambridge: Univ. Press 1970, p. 58
Burns, R.G.: Mixed valencies and site occupancies of iron in silicate minerals from Mössbauer spectroscopy. Can. J. Spect. 17, 51–59 (1972)
Burns, R.G., Simon, H.F.: Cation disorder in tourmalines. Geol. Soc. Amer. Ann. Mtg. Dallas, Abstr. 5, 563–564 (1973)
Burns, R.G., Strens, R.G.J.: Structural interpretation of polarized absorption spectra of the Al-Fe-Mn-Cr epidotes. Mineral. Mag. 36, 204–226 (1967)
Deer, W.A., Howie, R.A., Zussman, J.: Rock Forming Minerals. London: Longmans 1962, Vol. 1, pp. 300–319
Dowty, E., Clark, J.R.: Crystal structure refinement and optical properties of a Ti3+ fassaite from the Allende Meteorite. Am. Mineral. 58, 230–242 (1973)
Faye, G.H., Manning, P.G., Gosselin, J.R., Tremblay, R.J.: The optical absorption spectra of tourmaline: importance of charge-transfer processes. Can. Mineral. 12, 370–380 (1974)
Faye, G.H., Manning, P.G., Nickel, E.H.: The polarized optical absorption spectra of tourmaline, cordierite, chloritoid and vivianite: Ferrous-ferric electronic interaction as a source of pleochroism. Am. Mineral. 53, 1174–1201 (1968)
Ferguson, J., Fielding, P.E.: Origin of the colours of yellow, green and blue sapphires. Chem. Phys. Lett. 10, 262–265 (1971)
Ferguson, J., Fielding, P.E.: The origins of the colours of natural yellow, blue and green sapphires. Aust. J. Chem. 25, 1371–1385 (1972)
Ferguson, J., Krausz, E.R., Guggenheim, H.J.: MCD spectroscopy of transition metal ions in fluoride crystals. III: Fe2+ and Fe3+ in KZnF3. Molec. Phys. 29, 1785–1796 (1975)
Hermon, E., Simkin, D.J., Donnay, G., Muir, W.B.: The distribution of Fe2+ and Fe3+ in iron-bearing tourmalines: A Mössbauer study. Tschermaks Mineral. Petrogr. Mitt. 19, 124–132 (1973)
Lever, A.B.P.: Inorganic Electronic Spectroscopy. Amsterdam, London, New York: Elsevier 1968, pp. 146–149
Loeffler, B.M., Burns, R.G., Tossell, J.A.: Metal→metal charge transfer transitions: interpretation of visible-region spectra of the moon and lunar materials. Proc. 6th Lunar Sci. Conf., Geochim. Cosmochim. Acta, Suppl. 6, Vol. 3, 2663–2676 (1975)
Manning, P.G.: Absorption spectra of the manganese-bearing chain silicates, pyroxmangite, rhodonite, bustamite and serandite. Can. Mineral. 9, 348–357 (1968)
Manning, P.G.: An optical absorption study of the origin of colour and pleochroism in pink and brown tourmalines. Can. Mineral. 9, 678–690 (1969a)
Manning, P.G.: Optical absorption spectra of chromium-bearing tourmaline, black tourmaline and buergerite. Can. Mineral. 10, 57–70 (1969b)
Manning, P.G.: Optical absorption spectra of Fe3+ in octahedral and tetrahedral sites in natural garnets. Can. Mineral. 11, 826–839 (1972)
Price, D.C., Vance, E.R., Smith, G., Edgar, A., Dickson, B.L.: Mössbauer effect studies of beryl. J. de Phys., Colloque C6, suppl. 12, 37, 811–817 (1976)
Reed, S.J.B., Ware, N.G.: Quantitative electron microprobe analysis using a lithium drifted silicon detector. X-ray Spectrometry 2, 69–74 (1973)
Rossman, G.R.: Spectroscopic and magnetic studies of ferric iron hydroxy sulfates: the series Fe(OH)SO4·nH2O and the jarosites. Am. Mineral. 61, 398–404 (1976)
Samoilovich, M.I., Tsinober, L.I., Dunin-Barkovskii, R.L.: Nature of the coloring in iron-containing beryl. Kristallografiya 16, 186–189 (1971). [Sov. Phys. Cryst. 16, 147–150 (1971)]
Scorzelli, R.B., Baggio-Saitovich, E., Danon, J.: Mössbauer spectra and electron exchange in tourmaline and staurolite. J. de Phys., Colloque C6, suppl. 12, 37, 801–805 (1976)
Smith, G.: Polarized absorption spectra of transition-metal ions and ion-pairs in some ionic crystals. Ph. D. Thesis, University of Newcastle Upon Tyne, England (1973)
Smith, G.: Low temperature optical studies of metal-metal charge transfer transitions in various minerals. Can. Mineral. 15, 500–507 (1977)
Smith, G.: Evidence for absorption by exchange-coupled Fe2+-Fe3+ pairs in the near infra-red spectra of minerals. Phys. Chem. Minerals. 3, 375–383 (1978)
Smith, G., Strens, R.G.J.: Intervalence-transfer absorption in some silicate, oxide and phosphate minerals. In The Physics and Chemistry of Minerals and Rocks, Strens, R.G.J. (ed.). New York: J. Wiley and Sons 1976, pp. 583–612
Smith, G., Vance, E.R., Hasan, Z., Edgar, A., Runciman, W.A.: A charge transfer mechanism for the colour of rose quartz. Phys. Status Solidi (A) 46, K135-K140 (1978)
Townsend, M.G.: On the dichroism of tourmaline. J. Phys. Chem. Solids 31, 2481–2488 (1970)
Tsang, T., Thorpe, A.N., Donnay, G., Senftle, F.E.: Magnetic susceptibility and triangular exchange coupling in the tourmaline mineral group. J. Phys. Chem. Solids 32, 1441–1448 (1971)
White, W.B., Moore, R.K.: Interpretation of the spin-allowed bands of Fe2+ in silicate garnets. Am. Mineral. 57, 1692–1710 (1972)
Wilkins, R.W.T., Farrell, E.F., Naiman, C.S.: The crystal-field spectra and dichroism of tourmaline. J. Phys. Chem. Solids 30, 43–56 (1969)
Wood, D.L., Nassau, K.: The characterization of beryl and emerald by visible and infrared absorption spectroscopy. Am. Mineral 53, 777–800 (1968)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, G. A reassessment of the role of iron in the 5,000–30,000 cm−1 region of the electronic absorption spectra of tourmaline. Phys Chem Minerals 3, 343–373 (1978). https://doi.org/10.1007/BF00311847
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00311847