Abstract
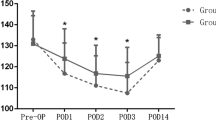
The risk of thrombosis after lower-extremity sclerotherapy is still an unresolved issue. This study was conducted to investigate the influence of sclerotherapy on coagulation and fibrinolysis by examining 20 patients who underwent surgical procedures, 10 of whom were treated by surgery alone (control group), while the other 10 were given sclerotherapy using 1% hydroxypolyaetoxydodecan as polidocanol (sclerotherapy group). Sex, age, and severity of disease was comparable between the two groups. No significant difference was found in the transient elevation of acute phase proteins, C-reactive protein (CRP), or fibrinogen. Thrombin antithrombin III complex (TAT), a marker of coagulation, transiently increased following treatment. In the control group, TAT peaked 3 days after treatment, whereas in the sclerotherapy group the elevation was prolonged, peaking 7 days after treatment. Elevation of the markers of fibrinolysis, plasmin plasmin inhibitor complex (PIC) and fibrin degradation products (FDP), was slower than that of TAT, peaking 7 days after treatment in both groups, the plasma PIC being significantly enhanced 7 days after treatment in the sclerotherapy group. A significant decrease in the platelet count was observed 3 days after treatment in the sclerotherapy group. These results suggest that sclerotherapy may enhance coagulation or fibrinolysis after surgical procedures.
Similar content being viewed by others
References
Goldman MP, Weiss RA, Bergan JJ (1994) Diagnosis and treatment of varicose veins: A review. J Am Acad Dermat 31:393–413
Crane C (1979) The surgery of varicose veins. Surg Clin North Am 59:737–748
Fegan WG (1963) Continuous compression technique of injecting varicose veins. Lancet 2:109–112
Feied CF (1993) Deep vein thrombosis: The risks of sclerotherapy in hypercoagulable state. Semin Dermatol 12:135–149
Van der Plas JPL, Lambers JCCA, Van Wersch JWJ, Koehler PJ (1994) Reversible ischaemic neurological deficit after sclerotherapy of varicose veins. Lancet 343:428–428
Cacciola E, Giustolisk R, Musso R (1987) Activation of contact phase of blood coagulation can be induced by the sclerosing agent polidocanol: possible additional mechanisms of adverse reaction during sclerotherapy. J Lab Clin Med 109:225–226
Wuppermann T, Hass KH (1975) The effect of sclerosing agent hydroxypolyaetoxydodecan on the coagulation potentials. In vitro investigations. Vasa 4:45–53
Wuppermann T (1977) Étude sur la sclérose des varices: Comparaison de la fibrinolyse naturelle dans le sang de la veine cubitale et du test au fibrinogène marqué dans la jambe sclérosée. Phlebologie 30:145–149
Suzuki N, Nakao A, Nonami T (1992) Experimental study on the effects of sclerosants for esophageal varices on blood coagulation, fibrinolysis and systemic hemodynamics. Gastroenterol Jpn 27:309–316
Baele G, De Vos M, Huble F (1984) Influence of injection sclerotherapy of esophageal varices in liver cirrhosis on the hemostatic system. Haemostasis 14:131–134
Hiemeyer V (1968) The fibrinolytic activity of blood derived from vessel wall. Angiologica 5:95–104
Latner AL (1947) Anxiety as a cause of fibrinolysis. Lancet 1:194–195
Perzer H, Schwarz A, Heimburger N (1988) Determination of human thrombin-antithrombin III complex in plasma with an enzyme-linked immunosorbent assay. Thromb Haemost 58:101–106
Harpel PC (1988) α2-plasmin inhibitor and α2-macroglobulin-plasmin complexes in plasma. J Clin Invest 68:46–55
Kang JH, Kambayashi J, Sakon M, Tsujinaka T, Mori T (1989) Postoperative changes in hemostasis analysed by the serial determination of fibrinopeptides and D-dimer. Jpn J Surg 19:262–268
Zimmerman JJ, Fogarty TJ (1983) Acute arterial occlusions. In: Moore WS (ed) Vascular surgery: A comprehensive review. Grune and Stratton, New York, pp 568–583
Brokemans AW, Veltkamp JJ, Bertina RM (1983) Congenital protein C deficiency and venous thromboembolism. A study of three Dutch families. N Engl J Med 309:340–341
Comp PC, Esmon CT (1984) Recurrent venous thromboem-bolism in patients with a partial deficiency of protein S. N Engl J Med 311:1525–1527
Dahlback B, Carlsson M, Svensson PJ (1993) Familiar thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C. Proc Natl Acad Sci USA 90:1004–1008
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ariyoshi, H., Kambayashi, Ji., Tominaga, S. et al. The possible risk of lower-limb sclerotherapy causing an extended hypercoagulable state. Surg Today 26, 323–327 (1996). https://doi.org/10.1007/BF00311600
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00311600