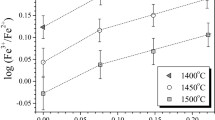
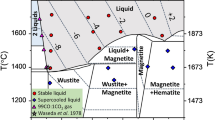
Abstract
Mossbauer spectroscopy has been used to determine the redox equilibria of iron and structure of quenched melts on the composition join Na2Si2O5-Fe2O3 to 40 kbar pressure at 1400° C. The Fe3+/ΣFe decreases with increasing pressure. The ferric iron appears to undergo a gradual coordination transformation from a network-former at 1 bar to a network-modifier at higher (≧10 kbar) pressure. Ferrous iron is a network-modifier in all quenched melts. Reduction of Fe3+ to Fe2+ and coordination transformation of remaining Fe3+ result in depolymerization of the silicate melts (the ratio of nonbridging oxygens per tetrahedral cations, NBO/T, increases). It is suggested that this pressure-induced depolymerization of iron-bearing silicate liquids results in increasing NBO/T of the liquidus minerals. Furthermore, this depolymerization results in a more rapid pressure-induced decrease in viscosity and activation energy of viscous flow of iron-bearing silicate melts than would be expected for iron-free silicate melts with similar NBO/T.
Similar content being viewed by others
References
Amthauer G, Annersten H, Hafner SS (1977) The Mossbauer spectrum of 57Fe in titanium-bearing andradites. Phys Chem Minerals 1:399–413
Amthauer G, Langer K, Schliestedt M (1980) Thermally activated electron delocalization in deerite. Phys Chem Minerals 6:19–30
Annersten H, Halenius H (1976) Iron distribution in pink muscovite: a discussion. Am Mineral 61:1045–1050
Annersten H, Olesch M (1978) Distribution of ferrous and ferric iron in clintonite and the Mossbauer characteristics of ferric iron in tetrahedral coordination. Can Mineral 16:199–204
Bell PM, Mao HK (1974) Crystal-field spectra of Fe2+ and Fe3+ in synthetic basalt glass as a function of oxygen fugacity. Carnegie Inst Washington Yearb 73:496–497
Bottinga Y, Richet P, Weill DF (1983) Calculation of the density and thermal expansion coefficients of silicate liquids. Bull Mineral 106:129–138
Bowen NL, Schairer JF, Willems HWM (1930) The ternary system Na2SiO3-Fe2O3-SiO2. Am J Sci 20:405–455
Boyd FR, England JL (1960) Apparatus for phase equilibrium measurements at pressures up to 50 kilobars and temperatures up to 1750° C. J Geophys Res 65:741–748
Brown GE, Keefer KD, Fenn PM (1978) Extended X-ray fine structure (EXAFS) study of iron-bearing silicate glass (abstr). Geol Soc Am Abstracts with Programs 10:373
Burnham CW (1981) The nature of multicomponent aluminosilicate melts. Phys Chem Earth 13–14:197–227
Calas G, Petiau J (1983) Structure of oxide glasses: spectroscopic studies of local order and crystallochemistry: geochemical implications. Bull Mineral 106:33–55
Calas G, Levitz P, Petiau J, Bondot P, Loupias G (1980) Étude de l'order local autoudu fer dans les verres silicates naturels et synthetiques at l'aide de la spectrometrie d'absorption. X Rev Phys Appl 15:1166–1167
Coey JMD, Moukarik A, McDonaugh CM (1982) Electron hopping in cronstedite. Solid State Commun 41:797–800
Danckwerth PA, Virgo D (1982) Structural state of Fe3+ in the system Na2O-SiO2-Fe-O. Carnegie Inst Washington Yearb 81:342–344
Eggler DH, Rosenhauer M (1978) Carbon dioxide in silicate melts. II. Solubilities of CO2 and H2O in CaMgSi2O6 (diopside) liquids and vapors at pressures to 40 kbar. Am J Sci 278:64–94
Eggler DH, Mysen BO, Hoering TC (1974) Gas species in sealed capsules in solid-media high-pressure apparatus. Carnegie Inst Washington Yearb 73:228–232
Eibschutz M, Lines MO, Nassau K (1980) Electric field gradient distribution in vitreous yttrium garnet. Phys Rev B 21:3767–3770
Evans BH, Amthauer G (1980) The electronic structure of ilvaite and the pressure and temperature dependence of its 57Fe Mossbauer spectrum. Phys Chem Solids 41:985–1001
Fox KE, Furukawa T, White WB (1982) Transition metal ions in silicate melts. Part 2. Iron in sodium disilicate glasses. Phys Chem Glasses 23:169–178
Fraser DG, Rammensee W, Jones RH (1983) The mixing properties of melts in the system NaAlSi2O6-KAlSi2O6 determined by Knudsen cell mass spectrometry. Bull Mineral 106:111–117
Hafner SS, Huckenholz HG (1971) Mossbauer spectrum of synthetic ferridiopside. Nature Phys Sci 233:255–261
Huggins Fe, Mao HK, Virgo D (1975) Mossbauer studies at high pressure. Carnegie Inst Washington Yearb 74:405–410
Lacey ED (1968) Structure transition in alkali silicate glasses. J Am Ceram Soc 51:150–157
Larson H, Chipman J (1953) Oxygen activity in iron oxide slags. Trans AIME 196:1089–1096
Mao HK, Virgo D, Bell PM (1973) Analytical study of the orange soil returned by the Apollo 17 astronauts. Carnegie Inst Washington Yearb 72:631–638
Merrill RB, Wyllie PJ (1973) Absorption of iron by platinum capsules in high-pressure rock-melting experiments. Am Mineral 58:16–20
Mo X, Carmichael ISE, Rivers M, Stebbins M (1982) The partial molar volume of Fe2O3 in multicomponent silicate glasses and the pressure dependence of oxygen fugacity in magmas. Mineral Mag 45:237–245
Mysen BO, Virgo D (1978) Influence of pressure, temperature, and bulk composition on melt structures in the system NaAlSi2O6-NaFe3+Si2O6. Am J Sci 278:1307–1322
Mysen BO, Virgo D (1983) Redox equilibria, structure and melt properties in the system Na2O-Al2O3-SiO2-Fe-O. Carnegie Inst Washington Yearb 82:313–317
Mysen BO, Seifert FA, Virgo D (1980) Structure and redox equilibria of iron-bearing silicate melts. Am Mineral 65:867–884
Mysen BO, Virgo D, Kushiro I (1981) The structural role of aluminum in silicate melts — a Raman spectroscopic study at 1 atmosphere. Am Mineral 66:678–701
Mysen BO, Virgo D, Seifert FA (1982A) The structure of silicate melts: implications for chemical and physical properties of natural magma. Rev Geophys 20:353–383
Mysen BO, Virgo D, Seifert FA (1982B) Distribution of aluminum between anionic units in depolymerized silicate melts as a function of pressure and temperature. Carnegie Inst Washington Yearb 81:360–366
Mysen BO, Virgo D, Seifert FA (1984) Redox equilibria of iron in alkaline earth silicate melts: Relationships between melt structure, oxygen fugacity, temperature and properties of iron-bearing silicate liquids. Am Mineral 69:834–847
Mysen BO, Carmichael ISE, Virgo D (1985A) A comparison of iron redox ratios in silicate glasses determined by wet-chemical and 57Fe Mossbauer resonant absorption methods. Contrib Mineral Petrol: in press
Mysen BO, Virgo D, Neumann ER, Seifert FA (1985B) Redox equilibria and the structural states of ferric and ferrous iron in melts in the system CaO-MgO-Al2O3-SiO2-Fi-O: relationships between redox equilibria, melt structure and liquidus phase equilibria. Am Mineral 70:317–330
Nolet DA, Burns RG (1979) Ilvaite: a study of temperature dependent delocalization by the Mossbauer effect. Phys Chem Minerals 4:221–234
Nolet DA, Burns RG, Flamm SL, Besancon JR (1979) Spectra of Fe-Ti silicates: implications to remote-sensing of planetary surfaces. Proc 10th Lunar Sci Conf: 1775–1786
O'Horo MP, Levy RA (1978) Effect of melt atmosphere on the magnetic properties of a [(SiO2)45(CaO)55]65[Fe2O3]35 glass. J Appl Phys 49:1635–1637
Osborn EF, Arculus RJ (1975) Phase relations in the system Mg2SiO4-iron oxide-CaAl2Si2O8-SiO2 at 10 kbar and their bearing on the origin of andesite. Carnegie Inst Washington Yearb 74:504–507
Paul A, Douglas RW (1965) Ferrous-ferric equilibrium in binary alkali silicate glasses. Phys Chem Glasses 6:207–211
Presnall DC, Brenner NL (1974) A method for studying iron silicate liquids under reducing conditions with negligible iron loss. 38:1785–1788
Sack RO, Carmichael ISE, Rivers M, Ghiorso MS (1980) Ferricferrous equilibria in natural silicate liquids at 1 bar. Contrib Mineral Petrol 75:369–377
Scarfe CM, Mysen BO, Virgo D (1979) Changes of viscosity and density of melts of sodium disilicate, sodium metasilicate and diopside composition with pressure. Carnegie Inst Washington Yearb 78:547–551
Tuthill RL, Sato M (1970) Phase relations of simulated lunar basalt as a function of oxygen fugacity and their bearing on the petrogenesis of the Apollo 11 basalts. Geochim Cosmochim Acta 34:1293–1302
Virgo D, Mysen BO (1985) The structural state of iron in oxidized vs reduced glasses at 1 atm: a 57Fe Mossbauer study. Phys Chem Minerals 12:17–86
Virgo D, Mysen BO, Danckwerth P, Seifert FA (1982) Speciation of Fe3+ in 1-atm Na2O-SiO2-Fe-O melts. Carnegie Inst Washington Yearb 81:349–352
Virgo D, Mysen BO, Danckwerth P (1983a) The coordination of Fe3+ in oxidized vs reduced sodium aluminosilicate glasses: a 57Fe Mossbauer study. Carnegie Inst Washington Yearb 82:309–313
Virgo D, Mysen BO, Danckwerth P (1983b) Redox equilibria and the anionic structure of Na2O xSiO2-Fe-O melts: effects of oxygen fugacity. Carnegie Inst Washington Yearb 82:305–309
Waychunas GA, Rossman GR (1983) Spectroscopic standard for tetrahedrally-coordinated ferric iron: γLiAlO2:Fe3+. Phys Chem Minerals 9:212–215
Wivel C, Morup S (1981) Improved computational procedure for evaluation of overlapping hyperfine parameter distributions in Mossbauer spectra. J Phys E: Sci Instrum 14:605–610
Waff HS (1977) The structural role of ferric iron in silicate melts. Can Mineral 15:198–199
Yoder HS Jr (1950) High-low quartz inversion up to 10,000 bars. Trans Am Geophys Union 31:827–835
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mysen, B.O., Virgo, D. Iron-bearing silicate melts: Relations between pressure and redox equilibria. Phys Chem Minerals 12, 191–200 (1985). https://doi.org/10.1007/BF00311288
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00311288