Summary
Heparan sulphate has been reported to be present in rat embryos. It is covalently linked to a core protein as heparan sulphate proteoglycan (HSPG). Heparitinase specifically degrades heparan sulphate, thus treatment of rat embryos with this enzyme in vitro should result in the perturbation of any tissue interactions which involve heparan sulphate proteoglycan. In this study heparitinase was either added to the culture medium or microinjected directly into the amniotic cavity.
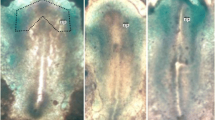
Heparitinase treatment resulted in abnormal development of the whole embryo, but the earliest effects were observed in the cranial region. Forebrain development was grossly abnormal: the neural folds remained widely open, with beak-like outgrowths rostrally. Optic sulci failed to develop. The midbrain and rostral hindbrain neural folds also remained widely open. In the trunk, where the pattern of neurulation is less complex than in the cranial region, rostral neural tube closure did occur although the morphology of the closed region was far from normal. These results suggest that heparan sulphate proteoglycan is essential for normal neurulation.
Epithelial somite formation was perturbed, but neural crest cell emigration, otic pit formation and pharyngeal arch formation, all important morphogenetic events which occur during this period of development, were not inhibited by heparitinase treatment. Prolonged (44h) exposure to the enzyme resulted in the conversion of the embryonic structure to a much simpler form: mesenchymal cells (stellate or spindle-shaped) enclosed within a simple epithelial coating.
Similar content being viewed by others
References
Banerjee SJ, Cohn RH, Bernfield MR (1977) The basal lamina of embryonic salivary epithelia: Production by the epithelium and role in maintaining lobular morphology. J Cell Biol 73:445–463
Beck F, Lloyd JB (1964) Dosage-response curves for the teratogenic activity of trypan blue. Nature 201:1136–1137
Bernfield MR, Banerier SD, Cohn RH (1972) Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide protein (proteoglycan) at the epithelial surface. J Cell Biol 52:674–689
Berry CL (1970) The effect of trypan blue on the growth of the rat embryo in vivo. J Embryol Exp Morphol 23:213–218
Biggers WJ, Barnea ER, Sanyal MK (1987) Anomalous neural differentiation induced by 5-Bromo-2′-deoxyuridine during organogenesis in the rat. Teratology 35:63–75
Chiarigi VP, Vannuchi S (1976) Surface heparan-sulphate as a control element in eukaryotic cells. J Theor Biol 61:459–475
Cockroft DL, Coppola PT (1977) Teratogenic effects of excess glucose on headfold rat embryos in culture. Teratology 16:141–146
Freeman SJ, Brown NA (1986) An in vitro study of teratogenicity in the rat due to antibody-induced yolk sac dysfunction. Roux's Arch Dev Biol 195:236–242
Fukunaga Y, Sobue M, Suzuki N, Kushida H, Suzuki S, Suzuki S (1975) Synthesis of a fluorogenic mucopolysaccharide by chondrocytes in cell culture with 4 methyl-umbelliferyl-β-d-xyloside. Biochim Biophys Acta 381:443–447
Gallagher JT, Hampson IN (1984) Proteoglycans in cellular differentiation and neoplasia. Biochem Soc Trans 12:541–543
Grief KF, Reichert LF (1982) Appearance and distribution of neuronal cell surface and synaptic vesicle antigens in the developing rat superior cervical ganglion. J Neurosci 2:843–852
Hook M, Kjellen L, Johansson S, Robinson J (1984) Cell-surface glycosaminoglycans. Ann Rev Biochem 53:847–869
Kjellen L, Oldberg A, Hook M (1980) Cell surface heparan sulfate. Mechanisms of proteoglycan cell association. J Biol Chem 255:10407–10413
Kraemer PM (1977) Heparin releases heparan sulfate from the cell surface. Biochem Biophys Res Commun 78:1334–1340
Krecht J, Cifonelli JA, Dorfman A (1967) Structural studies on heparitin sulphate of normal and Hurler tissues. J Biol Chem 242:4652–4661
Lander AD, Fujii DK, Gospodarowicz D, Reichardt LF (1982) Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulphate proteoglycan. J Cell Biol 94:574–585
Lindahl U, Hook M (1978) Glycosaminoglycans and their binding to biological macromolecules. Ann Rev Biochem 47:385–417
Morriss GM, New DAT (1979) Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. J Embryol Exp Morphol 54:17–35
Morriss GM, Solursh M (1978) Regional differences in mesenchymal cell morphology and glycosaminoglycans in early neural-fold stage rat embryos. J Embryol Exp Morphol 46:37–52
Morriss-Kay GM (1981) Growth and development of pattern in the cranial neural epithelium of rat embryos during neurulation. J Embryol Exp Morphol 65 [Suppl]:225–241
Morriss-Kay GM, Crutch B (1982) Culture of rat embryos with β-d-xyloside: evidence of a role for proteoglycans in neurulation. J Anat 134:491–506
Morriss-Kay GM, Tuckett F (1985) The role of microfilaments in cranial neurulation in rat embryos: effects of short-term exposure to cytochalasin D. J Embryol Exp Morphol 88:333–348
Morriss-Kay GM, Tuckett F (1987) Fluidity of the neural epithelium during forebrain formation in rat embryos. J Cell Sci [Suppl] 8:433–449
Morriss-Kay GM, Tuckett F (1989) Immunohistochemical localisation of chondroitin sulphate proteoglycans and the effects of Chondroitinase ABC in 9-to 11-day rat embryos. Development 106:787–798
Morriss-Kay GM, Tuckett F, Solursh M (1986) The effects of Streptomyces hyaluronidase on tissue organization and cell cycle time in rat embryos. J Embryol Exp Morphol 98:59–70
Pinter E, Reece EA, Leranth CZ, Sanyal MK, Hobbins JC, Mahoney MJ, Naftolin F (1986) Yolk sac failure in embryopathy due to hyperglycemia: ultrastructural analysis of yolk sac differentiation associated with embryopathy in rat concepturses under hyperglycemic conditions. Teratology 33:73–84
Rapraeger A, Bernfield MR (1985) Cell surface proteoglycan of mouse mammary epithelial cells: protease releases a heparan sulfate-rich ectodomain from a putative membrane-anchored domain. J Biol Chem 260:4103–4109
Ratner N, Bunge RP, Glaser L (1985) A neuronal cell surface heparan sulfate proteoglycan is required for dorsal root ganglion neuron stimulation of Schwann cell proliferation. J Cell Biol 101:744–754
Sainte-Marie G (1962) A paraffin embedding technique for studies employing immunofluoresence. J Histochem 10:250–256
Schluter G (1978) Ultrastructural changes of the early visceral yolk sac layer of mouse embryos after maternal injection of trypan blue. Anat Embryol 153:287–293
Schwartz NB (1977) Regulation of chondroitin sulfate synthesis. Effect of β-d-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains and core protein. J Biol Chem 252:6316–6321
Solursh M, Morriss GM (1977) Glycosaminoglycan synthesis in rat embryos during the formation of the primary mesenchyme and neural folds. Dev Biol 57:75–86
Thompson HA, Spooner BS (1982) Inhibition of branching morphogenesis and alteration of glycosaminoglycan biosynthesis in salivary glands treated with β-d-xyloside. Dev Biol 89:417–424
Thompson HA, Spooner BS (1983) Proteoglycan and glycosaminoglycan synthesis in embryonic salivary glands: Effects of β-d-xyloside, an inhibitor of branching morphogenesis. J Cell Biol 96:1443–1450
Trelstad RL (1984) The Role of Extracellular Matrix in Development. Alan R Liss, New York
Tuckett F, Morriss-Kay GM (1985) The kinetic behaviour of the cranial neural epithelium during neurulation in the rat. J Embryol Exp Morphol 85:111–119
Tuckett F, Morriss-Kay GM (1986) The distribution of fibronectin laminin and entactin in the neurulating rat embryo studied by indirect immunofluorescence. J Embryol Exp Morphol 94:95–112
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tuckett, F., Morriss-Kay, G.M. Heparitinase treatment of rat embryos during cranial neurulation. Anat Embryol 180, 393–400 (1989). https://doi.org/10.1007/BF00311170
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00311170