Abstract
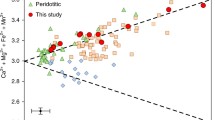
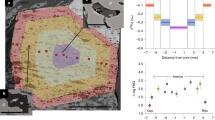
The oxidation state of a mantle assemblage may be defined by heterogeneous reactions between oxygen and iron-bearing minerals. In spinel lherzolites, the presence of Fe3+ in spinel allows use of the assemblage olivine-orthopyroxene-spinel to define f O 2 at fixed T and P. As a first step towards establishing an analogous reaction for garnet lherzolites, garnets from mantle-derived xenoliths from South Africa and the USSR have been analyzed with 57Fe Mössbauer spectroscopy at 298 and 77K to determine Fe3+/Fe2+ and the coordination state of iron. Garnets from South African alkremites (pyrope+Mg-spinel) and eclogites, as well as garnet-spinel and low-temperature garnet lherzolites from both South Afica and the USSR, have Fe3+/ΣFe<0.07. In contrast, garnets from high-temperature garnet lherzolites from within the Kaapvaal craton of South Africa have Fe3+/ΣFe>0.10. Ferric iron is octahedrally coordinated, and ferrous iron is present in the dodecahedral site in all samples. The occurrence of significant Fe3+ in these garnets necessitates caution in the use of geothermometers and geobarometers that are applied to mantle samples. For example, the presence of ∼12% of the Fe as Fe3+ in garnets can increase temperatures calculated from existing Fe/Mg geothermometers by>200°C. The concomitant increase in pressures calculated from geobarometers that use the Al content in orthopyroxene coexisting with garnet are ∼10–15 kbar. Results of calculations based on heterogeneous equilibria between garnet, olivine, and pyroxene are consistent with the derivation of the peridotite samples from source regions that are relatively oxidized, between the f O 2 of the FMQ (quartz-fayalite-magnetite) buffer and that of the WM buffer. No samples yield values of f O 2 as reduced as IW (iron-wüstite buffer).
Similar content being viewed by others
References
Albee AL, Ray L (1970) Correction factors for electron probe microanalysis of silicates, oxides, carbonates, phosphates, and sulfates. Anal Chem 42:1408–1414
Aldridge LP, Bancroft GM, Fleet ME, Herzberg CT (1978) Omphacite studies, II. Mössbauer spectra of C2/c and P2/n omphacites. Am Mineral 63:1107–1115
Amthauer G, Annersten H, Hafner SS (1976) The Mössbauer spectrum of 57Fe in silicate garnets. Z Kristallogr 143:14–55
Arculus RJ (1985) Oxidation status of the mantle, past and present. Ann Rev Earth Planet Sci 13:75–95
Arculus RJ, Delano JW (1981) Intrinsic oxygen fugacity measurements: techniques and results for spinels from upper mantle peridotites and megacryst assemblages. Geochim Cosmochim Acta 45:899–913
Arculus RJ, Dawson JB, Mitchell RH, Gust DA, Holmes RD (1984) Oxidation states of the upper mantle recorded by megacryst ilmenite in kimberlite and type A and B spinel lherzolites. Contrib Mineral Petrol 85:85–94
Bancroft GM (1973) Mössbauer spectroscopy: an introduction for inorganic chemists and geochemists. Wiley, New York, 252 p
Bancroft GM, Osborne MD, Fleet ME (1983) Next-nearest-neighbour effects in the Mössbauer spectra of Cr-spinels: an application of partial quadrupole splittings. Sol State Comm 47:623–625
Bence AE, Albee AL (1968) Empirical correction factors for the electron microanalysis of silicates and oxides. J Geol 76:382–403
Bertrand P, Mercier J-CC (1985) The mutual solubility of coexisting ortho-and clinopyroxene: towards an absolute geothermometer for the natural system? Earth Planet Sci Lett 76:109–122
Boyd FR, Mertzman SA (1987) Composition and structure of the Kaapvaal lithosphere, Southern Africa. In: Mysen BO (ed) Magmatic processes: physicochemical principles. Geochem Soc Spec Publ 1:13–24
Boyd FR, Gurney JJ, Richardson SH (1985) Evidence for a 150–200 km thick Archaean lithosphere from diamond inclusion thermobarometry. Nature 315:387–389
Christie DM, Carmichael ISE, Langmuir CH (1986) Oxidation states of mid-ocean ridge basalt galsses. Earth Planet Sci Lett 79:397–411
Cox KG, Smith MR, Beswetherick S (1987) Textural studies of garnet lherzolites: evidence of exsolution origin from high-temperature harzburgites. In: Nixon PH (ed) Mantle xenoliths. Wiley, New York, pp 537–550
Davidson WC (1959) Variable metric method for minimization. Argonne National Laboratory, ANL 5990
Dollase WA (1975) Statistical limitations of Mössbauer spectral fitting. Am Mineral 60:257–264
Dollase WA, Gustafson WI (1982) 57Fe Mössbauer spectral analysis of the sodic clinopyroxenes. Am Mineral 67:311–327
Dowty E, Lindsley DH (1973) Mössbauer spectra of synthetic hedenbergite-ferrosilite pyroxenes. Am Mineral 58:850–868
Finnerty AA, Boyd FR (1987) Thermobarometry for garnet peridotites: basis for the determination of thermal and compositional structure of the upper mantle. In: Nixon PH (ed) Mantle xenoliths. Wiley, New York, 381–402
Haggerty SE, Tompkins LA (1983) Redox state of Earth's upper mantle from kimberlitic ilmenites. Nature 303:295–300
Hatton CJ (1988) Geochemistry and origin of xenoliths from the Roberts Victor Mine. PhD thesis, Dept of Geochemistry, Univ Cape Town
Harley SL (1984) An experimental study of the partitioning of Fe and Mg between garnet and orthopyroxene. Contrib Mineral Petrol 86:359–373
Helffrich G, Wood BJ (1989) Subregular model for multicomponent solutions. Am Mineral. (In press)
Luth RW, Virgo D, Boyd FR, Wood BJ (1988) Valence and coordination of Fe in mantle-derived garnets. GSA Abst with Progr 20:A101–102
Lyubutin IS, Dodokin AP (1971a) Temperature dependence of the Mössbauer effect for tetrahedral iron atoms in garnet. Sov Phys Crystallogr 15:936–938
Lyubutin IS, Dodokin AP (1971b) Temperature depedence of the Mössbauer effect for Fe2+ in dodecahedral coordination in garnet. Sov Phys Crystallogr 15:1091–1092
MacGregor ID (1974) The system MgO-Al2-SiO2: solubility of Al2O3 in entstatite for spinel and garnet peridotite compositions. Am Mineral 61:603–615
Mattioli GS, Bishop FC (1984) Experimental determination of the chromium-aluminum mixing parameter in garnet. Geochim Cosmochim Acta 48:1367–1371
Mattioli GS, Wood BJ (1986) Upper mantle oxygen fugacity recorded by spinel-lherzolites. Nature 322:626–628
Moecher DP, Essene EJ, Anovitz LM (1988) Calculation and application of clinopyroxene—garnet—plagioclase—quartz geobarometers. Contrib Mineral Petrol 100:92–106
Murad E, Wagner FE (1987) The Mössbauer spectrum of almandine. Phys Chem Mineral 14:264–269
Navrotsky A (1987) Models of crystalline solutions. In: Carmichael ISE, Eguster HP (eds) Thermodynamic modeling of geological materials: minerals, fluids, and melts. Rev Mineral 17:35–69, Mineral Soc Am
Nickel KG, Green DH (1985) Empirical geothermobarometry for garnet peridotites and implications for the nature of the lithosphere, kimberlites and diamonds. Earth Planet Sci Lett 73:158–170
Nixon PH, Boyd FR (1973) Petrogenesis of the granular and sheared ultrabasic nodule suite in kimberlite. In: Nixon PH (ed) Lesotho Kimberlites. Lesotho National Development Corp, Maseru, pp 48–56
Nixon PH, Chapman NA, Gurney JJ (1978) Pyrope-spine (alkremite) xenoliths from kimberlite. Contrib Mineral Petrol 65:341–346
Nixon PH, van Calsteren PWC, Boyd FR, Hawkesworth CJ (1987) Harzburgites with garnets of diamond facies from southern African kimberlites. In: Nixon PH (ed) Mantle xenoliths. Wiley, New York, pp 523–533
O'Hara MJ, Saunders MJ, Mercy ELP (1975) Garnet-peridotite, primary ultrabasic magma and eclogite; interpretation of upper mantle processes in kimberlite. Phys Chem Earth 9:571–604
O'Neill HStC, Wall VJ (1987) The olivine-orthopyroxene-spinel oxygen geobarometer, the nickel precipitation curve, and the oxygen fugacity of the Earth's upper mantle J Petrol 28:1169–1191
O'Neill HStC, Wood BJ (1979) An experimental study of Fe-Mg partitioning between garnet and olivine and its calibration as a geothermometer. Contrib Mineral Petrol 70:59–70
O'Neill HStC, Ortez N, Arculus RJ, Wall VJ, Green DH (1982) Oxygen fugacities from the assemblage olivine-orthopyroxene-spinel (Abstr). Terra Cognita 2:228
Ruby SL (1973) Why MISFIT when you already have x 2? In: Gruverman IJ, Seidel CW (eds) Mössbauer effect methodology, vol 8. Plenum Press, New York, pp 263–276
Seifert F (1983) Mössbauer line broadening in aluminous orthopyroxenes: Evidence for next nearest neighbors interactions and short-range order. Neues Jahrb Mineral Abh 148:141–162
Smith D, Boyd FR (1987) Compositional heterogeneities in a hightemperature lherzolite nodule and implications for mantle processes. In: Nixon PH (ed) Mantle xenoliths. Wiley, New York, pp 551–561
Stevens IG, Stevens VE (1972) Mössbauer Effect Data Index, covering the 1970 literature IFI/Plenum Data Corporation
Ulmer GC, Grandstaff DE, Weiss D, Moats MA, Buntin TJ, Gold DP, Hatton CJ, Kadik A, Koseluk RA, Rosenhauer M (1987) The mantle redox story: an unfinished story. In: Mantle metasomatism and alkaline magmatism. Geol Soc Am Spec Pap 215, pp 5–23
Virgo D, Hafner SS (1969) Fe2+-Mg order-disorder in heated orthopyroxenes. Mineral Soc Am Spec Pap 2:67–81
Virgo D, Luth RW, Moats MA, Ulmer GC (1988) Constraints on the oxidation state of the mantle: an electrochemical and 57Fe Mössbauer study of mantle-derived ilmenites. Geochim Cosmochim Acta 52:1781–1794
Waychunas GA (1986) Performance and use of Mössbauer good-ness-of-fit parameters: response to spectra of varying signal/noise ratio and possible misinterpretations. Am Mineral 71:1261–1265
Webb SAC, Wood BJ (1986) Spinel-pyroxene-garnet relationships and their dependence on Cr/Al ratio. Contrib Mineral Petrol 92:471–480
Wells PRA (1977) Pyroxene thermometry in simple and complex systems. Contrib Mineral Petrol 62:129–139
Wood BJ (1987)Thermodynamics of multicomponent systems containing several solid solutions. In: Carmichael ISE, Eugster HP (eds) Thermodynamic modeling of geological materials: minerals, fluids, and melts. Rev Mineral 17:71–95, Mineral Soc Am
Wood BJ, Banno S (1973) Garnet-orthopyroxene and orthopyroxene-clinopyroxene relationships in simple and complex systems. Contrib Mineral Petrol 42:109–124
Wood BJ, Nicholls J (1978) The thermodynamic properties of reciprocal solid solutions. Contrib Mineral Petrol 66:389–400
Wood BJ, Virgo D (1989) Upper mantle oxidation state: Ferric iron contents of lherzolite spinels by 57Fe Mössbauer spectroscopy and resultant oxygen fugacities. Geochim Cosmochim Acta 53:1277–1291
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luth, R.W., Virgo, D., Boyd, F.R. et al. Ferric iron in mantle-derived garnets. Contr. Mineral. and Petrol. 104, 56–72 (1990). https://doi.org/10.1007/BF00310646
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00310646