Abstract
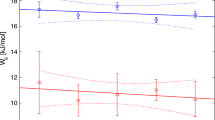
Variation in published alkali-feldspar solvus curves is discussed in the light of the concept of complete (crystal-crystal) and exchange (crystal-fluid-crystal) equilibrium. Exchange equilibrium may lead to very regular solvus-like two-phase curves differing substantially from the true binodal solvus; certain experimental strategies tend to favour the attainment and persistence of the exchange equilibrium condition. New long duration experiments under “alkali excess” and “alkali+silica excess” conditions did not yield fledspar pairs significantly off the bracketed binodal obtained by Smith and Parsons (1974) but exchange equilibrium behaviour is shown to influence the course of equilibration in peralkaline experiments. Agreement in solvus curves obtained by bracketing experiments by several workers is excellent (±2 mol. % Or) with a straight linear relationship between T crit and P such that dT crit/dP≈16°/Kbar. These curves do not exhibit breaks or sensitivity to chemical environment, and there is no evidence that the solvus changes shape with increasing P.
Similar content being viewed by others
References
Bachinski, S.W., Müller, G.: Experimental determinations of the microcline-low albite solvus. J. Petrol. 12, 329–356 (1971)
Currie, K.L.: On the solubility of albite in supercritical water in the range 400 to 600°C and 750 to 3500 bars. Am. J. Sci., 266, 321–341 (1968)
Eugster, H.P., Albee, A.L., Bence, A.E., Thompson, J.B., Jr., Waldbaum, D.R.: The two-phase region and excess mixing properties of paragonite-muscovite crystalline solutions. J. Petrol., 13, 147–179 (1972)
Goldsmith, J.R., Newton, R.C.: An experimental determination of the alkali feldspar solvus. In: The feldspars, MacKenzie, W.S. and Zussman, J. (eds.), Manchester: Manchster University Press 1974, pp. 337–359
Lagache, M., Weisbrod, A.: The system: Two alkali Feldspars-KCl-NaCl-H2O at moderate to high temperatures and low pressures. Contrib. Mineral. Petrol., 62, 77–102 (1977)
Luth, W.C., Martin, R.F., Fenn, P.M.: Peralkaline alkali feldspar solvi. In: The feldspars, MacKenzie, W.S. and Zussman, J. (eds.). Manchester: Manchester University Press 1974, pp. 297–312
Luth, W.C., Querol-Suñé, F.: An alkali feldspar series. Contrib. Mineral. Petrol., 25, 25–40 (1970)
Luft, W.C., Tuttle, O.F.: The alkali feldspar solvus in the system Na2O-K2O-Al2O3-SiO2-H2O. Am. Mineral. 51, 1359–1373 (1966)
Martin, R.F.: The alkali feldspar solvus: the case for a first order break on the K-limb. Bull. Soc. fr. Minéral. Cristallogr., 97, 346–355 (1974)
Mason, R.A., Parsons, I.: New data on the stability of low albite and the effect of ordering on the alkali feldspar solvus. Mineral. Soc. Bull. 30, 9 (1976)
Morse, S.A.: Alkali feldspars with water at 5 kb pressure. J. Petrol. 11, 221–253 (1970)
Orville, P.M.: Alkali ion exchange between vapor and feldspar phases. Am. J. Sci. 261, 201–237 (1963)
Parsons, I.: An experimental study of ordering in sodium-rich alkali feldspars. Mineral. Mag., 36, 1061–1077 (1968)
Parsons, I.: Subsolidus crystallization behaviour in the system KAlSi3O8-NaAlSi3O8-H2O. Mineral. Mag., 37, 173–180 (1969)
Seck, H.A.: The influence of pressure on the alkali-feldspar solvus from peraluminous and persilicic materials. Fortschr. Mineral. 49, 31–49 (1972)
Senderov, E.E., Shchekina, T.I., Tobelko, K.I.: A study of low albite crystallization (in Russian). Geokhimiya 8, 35–43 (1971)
Smith, P.: Subsolidus crystallization in the system NaAlSiO8(Ab)-CaAl2Si2O8(An)-KAlSi3O8(Or) at PH2O 1 kilobar. Unpubl. Ph.D. Thesis, Univ. of Aberdeen (1974)
Smith, P., Parsons, I.: The alkali-feldspar solvus at 1 kilobar water vapour pressure. Mineral. Mag. 39, 747–767 (1974)
Stewart, D.B., Ribbe, P.H.: Structural explanation for variations in cell parameters of alkali feldspar with Al/Si ordering. Am. J. Sci. 267-A, 444–462 (1969)
Thompson, J.B.Jr., Waldbaum, D.R.: Mixing properties of sanidine crystalline solutions. I. Calculations based on ion-exchange data. Am. Mineral. 53, 1965–1999 (1968)
Thompson, J.B.Jr., Waldbaum, D.R.: Mixing properties of sanidine crystalline solutions. III. Calculations based on two-phase data. Am. Mineral. 54, 811–838 (1969)
Thompson, R.N., MacKenzie, W.S.: Feldspar-liquid equilibria in peralkaline acid liquids: an experimental study. Am. J. Sci. 265, 714–734 (1967)
Trembath, L.T.: Hydrothermal synthesis of albite: the effect of NaOH on obliquity. Mineral. Mag. 39, 455–463 (1973)
Tuttle, O.F., Bowen, N.L.: Origin of granite in the light of experimental studies. Geol. Soc. Am. Mem. 74 (1958)
Waldbaum, D.B., Thompson, J.B.Jr.: Mixing properties of sanidine crystalline solutions. IV. Phase diagrams from equations of state. Am. Mineral., 54, 1274–1298 (1969)
Wright, T.L.: X-ray and optical study of alkali feldspar. II. An X-ray method for determining the composition and structural state from measurements of 2-theta values for three reflections. Am. Mineral., 53, 88–104 (1968)
Yund, R.A.: Microstructure, kinetics and mechanisms of alkali feldspar exsolution. In: Feldspar mineralogy, Ribbe, P.H. (ed.). Min. Soc. Am. Short Course Notes, 2, Y29-Y57 (1975)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parsons, I. Alkali-feldspars: Which solvus?. Phys Chem Minerals 2, 199–213 (1978). https://doi.org/10.1007/BF00308173
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00308173