Abstract
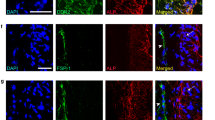
CD44 is a multifunctional adhesion molecule that binds to hyaluronic acid, type I collagen, and fibronectin. We have studied the immunohistochemical localization of CD44 in bone cells by confocal laser scanning microscopy and transmission electron microscopy in order to clarify its role in the cell-cell and/or cell-matrix interaction of bone cells. In round osteoblasts attached to bone surfaces, immunoreactivity is restricted to their cytoplasmic processes. On the other hand, osteocytes in bone matrices show intense immunoreactivity on their plasma membrane. Intense immunoreactivity for CD44 can be detected on the basolateral plasma membranes of osteoclasts. There is considerably less reactivity observed in the area of the plasma membrane that is in direct contact with bone. The pre-embedding electron-microscopical method has revealed that CD44 is mainly localized on the basolateral plasma membrane of osteoclasts. However, the ruffled border and clear zone show little immunoreactivity. A CD44-positive reaction can be detected on both plasma membranes in the contact region between osteoclasts and osteocytes. These findings suggest that: 1) cells of the osteoblast lineage express CD44 in accordance with their morphological changes from osteoblasts into osteocytes; 2) osteoclasts express CD44 on their basolateral plasma membrane; 3) CD44 in osteoclasts and osteocytes may play an important role in cell-cell and/or cell-matrix attachment via extracellular matrices.
Similar content being viewed by others
References
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61:1303–1313
Aubin JE, Heersche JNM, Merrilees MJ, Sodek J (1982) Isolation of bone cell clones with differences in growth, hormone responses, and extracellular matrix production. J Cell Biol 92:452–461
Brown TA, Bouchard T, John TS, Wayner E, Carter WG (1991) Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol 113:207–221
Carter WG, Wayner EA (1988) Characterization of the class III collagen receptor, a phosphorylated, transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem 263:4193–4201
Cochran DL (1987) Glycosaminoglycan stimulation of calcium release from mouse calvariae. Specificity for hyaluronic acid and dermatan sulfate. Calcif Tissue Int 41:79–85
Davies J, Warwick J, Totty N, Philp R, Helfrich M, Horton M (1989) The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol 109:1817–1826
Ejiri S (1983) The preosteoclast and its cytodifferentiation into the osteoclast: ultrastructural and histochemical studies of rat fetal parietal bone. Arch Histol Jpn 46:533–557
Faassen AE, Schrager JA, Klein DJ, Oegema TR, Couchman JR, McCarthy JB (1992) A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J Cell Biol 116:521–531
Fedarko NS, Termine JD, Young MF, Robey PG (1990) Temporal regulation of hyaluronan and proteoglycan metabolism by human bone cells in vitro. J Biol Chem 265:12200–12209
Goldstein LA, Zhou DFH, Picker LJ, Minty CN, Bargatze RF, Ding JF, Butcher EC (1989) A human lymphocyte homing receptor, the Hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell 56:1063–1072
Gordon MY, Riley GP, Watt SM, Greaves MF (1987) Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature 326:403–405
Güthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haußmann I, Ponta H, Herrlich P (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65:13–24
Haynes BF, Liao HX, Patton KL (1981) The transimembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells 3:347–350
Huet S, Groux H, Caillou B, Valentin H, Prieur M, Bernard A (1989) CD44 contributes to T cell activation. J Immunol 143:798–801
Hughes EN, Colombatti A, August JT (1983) Murine cell surface glycoproteins. Purification of the polymorphic Pgp-1 antigen and analysis of its expression on macrophages and other myeloid cells. J Biol Chem 258:1014–1021
Hughes DE, Salter DM, Simpson R (1994) CD44 expression in human bone: a novel marker of osteocytic differentiation. J Bone Miner Res 9:39–44
Hynes RO (1987) Integrins: a family of cell surface receptors. Cell 48:549–554
Irie K, Ozawa H (1990) Relationships between tooth eruption, occlusion and alveolar bone resorption: cytological and cytochemical studies of bone resorption on rat incisor alveolar bone facing the enamel. Arch Histol Cytol 53:497–509
Jalkanen S, Jalkanen M (1992) Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol 116:817–825
Jalkanen S, Bargatze RF, Herron LR, Butcher EC (1986) A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol 16:1195–1202
Jalkanen S, Bargatze RF, Toyos J, Butcher EC (1987) Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95 kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol 105:983–990
Jacobson K, O'dell D, Holifield B, Murphy TL, August JT (1984) Redistribution of a major cell surface glycoprotein during cell movement. J Cell Biol 99:1613–1623
Kalomiris EL, Bourguignon LYW (1988) Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol 106:319–327
Kennel SJ, Lankford TK, Foote LJ, Shinpock SG, Stringer C (1993) CD44 expression on murine tissues. J Cell Sci 104:373–382
Kodama H, Nose M, Niida S, Yamasaki A (1991) Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med 173:1291–1294
Koopman G, Kooyk YV, Graaff MD, Meyer CJLM, Figdor CG, Pals ST (1990) Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol 145:3589–3593
Kurihara N, Suda T, Miura Y, Nakauchi H, Kodama H, Hiura K, Hakeda Y, Kumegawa M (1989) Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood 74:1295–1302
Lacy BE, Underhill CB (1987) The hyaluronate receptor is associated with actin filaments. J Cell Biol 105:1395–1404
Lakkakorpi PT, Horton MA, Helfrich MH, Karhukorpi E-K, Väänänen HK (1991) Vitronectin receptor has a role in bone resorption but does not mediate tight sealing zone attachment of osteoclasts to the bone surface. J Cell Biol 115:1179–1186
Miyake K, Underhill CB, Lesley J, Kincade PW (1990a) Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med 172:69–75
Miyake K, Medina KL, Hayashi S, Ono S, Hamaoka T, Kincade PW (1990b) Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med 171:477–488
Miyauchi A, Alvarez J, Greenfield EM, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross FP, Teitelbaum S L, Cheresh D, Hruska KA (1991) Recognition of osteopontin and related peptides by an αVβ3integrin stimulates immediate cell signals in osteoclasts. J Biol Chem 266:20369–20374
Mundy GR, Roodman GR (1987) Osteoclast ontogeny and function. In: Peck WA (ed) Bone and mineral research, vol 5. Elsevier, Amsterdam, pp 209–279
Nakamura H, Ozawa H (1994) Immunohistochemical localization of heparan sulfate proteoglycan in rat tibiae. J Bone Miner Res 9:1289–1299
Reinholt FP, Hultenby K, Oldberg A, Heinegard D (1990) Osteopontin—a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA 87:4473–4475
Roberts R, Gallagher J, Spooncer E, Allen TD, Bloomfield F, Dexter TM (1988) Heparan sulfate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature 332:376–378
Ruoslahti E, Yamaguchi Y (1991) Proteoglycans as modulators of growth factor activities. Cell 64:867–869
Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI (1992) Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 89:12160–12164
Shimizu Y, Seventer GAV, Siraganian R, Wahl L, Shaw S (1989) Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol 143:2457–2463
Stamenkovic I, Amiot M, Pesando JM, Seed B (1989) A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 56:1057–1062
Takagi M, Parmley RT, Toda Y, Denys FR (1982) Extracellular and intracellular digestion of complex carbohydrates by osteoclasts. Lab Invest 46:288–297
Takagi M, Parmley RT, Toda Y, Denys FR (1983) Ultrastructural cytochemistry of complex carbohydrates in osteoblasts, osteoid and bone matrix. Calcif Tissue Int 35:309–319
Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123:2600–2602
Takeichi M (1988) The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 103:639–655
Tarone G, Ferracini R, Galetto G, Comoglio P (1984) A cell surface integral membrane glycoprotein of 85,000 mol wt (gp85) associated with Triton X-100-insoluble cell skeleton. J Cell Biol 99:512–519
Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T (1989) The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology 125:1805–1813
Underhill C (1992) CD44: the hyaluronan receptor. J Cell Sci 103:293–298
Wolffe EJ, Gause WC, Pelfrey CM, Holland SM, Steinberg AD, August JT (1990) The cDNA sequence of mouse Pgp-1 and homology to human CD44 cell surface antigen and proteoglycan core/link proteins. J Biol Chem 265:341–347
Wong GL (1984) Paracrine interactions in bone-secreted products of osteoblasts permit osteoclasts to respond to parathyroid hormone. J Biol Chem 259:4019–4022
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakamura, H., Kenmotsu, Si., Sakai, H. et al. Localization of CD44, the hyaluronate receptor; on the plasma membrane of osteocytes and osteoclasts in rat tibiae. Cell Tissue Res 280, 225–233 (1995). https://doi.org/10.1007/BF00307793
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00307793