Summary
A comparison of rectal morphology and ultrastructure is made between a freshwater (A. aegypti) and salt water (A. campestris) species of mosquito larvae, and between A. campestris larvae producing hyper- and hyposmotic urine.
The epithelium of A. aegypti contains one cell type characterized by infolding of both the apical and basal membranes, straight lateral borders, and evenly distributed mitochondria.
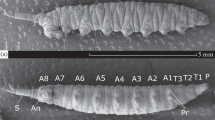
The rectum of A. campestris contains distinct anterior and posterior regions, each made up of a single cell type. These two regions can be distinguished on the basis of cell thickness, depth of apical infolding and distribution of mitochondria. The anterior region is similar to the rectum of A. aegypti, while the posterior region is considered unique to the salt-water species and hence probably is associated with the formation of hyperosmotic urine.
In A. campestris, the apical (rather than lateral or basal) membranes are probably the site of hyperosmotic urine production. Two possible mechanisms for this process are discussed.
Similar content being viewed by others
References
Asakura, K.: Studies on the structural basis for ion- and water-transport by the osmoregulatory organs of mosquitoes. I. Fine structure of the rectal epithelium of Aedes albopictus larva. Sci. Rep. Kanazawa Univ. 1, 37–55 (1970).
Beadle, L. C.: Regulation of the hemolymph in the saline water mosquito larvae Aedes detritus Edw. J. exp. Biol. 16, 346–362 (1939).
Berridge, M. J., Gupta, B. L.: Fine structural changes in relation to ion and water transport in the rectal papillae of the blowfly Calliphora. J. Cell Sci. 2, 89–112 (1967).
Copeland, E.: A mitochondrial pump in the cells of the anal papillae of mosquito larvae. J. Cell Biol. 23, 253–264 (1964).
Gomori, G.: Observations with differential stains on human islets of Langerhans. Amer. J. Path. 17, 395–406 (1941).
Goodchild, A. J. P.: The rectal glands of Halosalada lateralis (Fallén) (Hemiptera: Saldidae) and Hydrometra stagnorum (L.) (Hemiptera: Hydrometridae). Proc. roy. ent. Soc. Lond. (A) 44, 62–70 (1969).
Grimstone, A. V., Mullinger, A. M., Ramsay, J. A.: Further studies on the rectal complex of the mealworm Tenebrio molitor L. (Coleoptera, Tenebrionidae). Phil. Trans. B 253, 342–382 (1968).
Gupta, B. L., Berridge, M. J.: Fine structural organization of the rectum in the blowfly, Calliphora erythrocephala (Meig.) with special reference to connective tissue, tracheae, and neurosecretory innervation in the rectal papillae. J. Morph. 120, 23–82 (1966a).
Gupta, B. L., Berridge, M. J.: A coat of repeating subunits on the cytoplasmic surface of the plasma membrane in the rectal papillae of the blowfly, Calliphora erythrocephala (Meig.), studied in situ by electron microscopy. J. Cell Biol. 29, 376–382 (1966b).
Hopkins, C. R.: The fine-structural changes observed in the rectal papillae of the mosquito Aedes aegypti L. and their relation to epithelial transport of water and inorganic ions. J. roy. micr. Soc. 86, 235–252 (1967).
Jarial, M. S., Scudder, G. G. E.: The morphology and ultrastructure of the Malpighian tubules and hindgut in Cenocorixa bifida (Hung.) (Hemiptera, Corixidae). Z. Morph. Ökol. Tiere 68, 269–299 (1970).
Maddrell, S. H. P.: The mechanisms of insect excretory systems. Advanc. Insect Physiol. 8, 199–331 (1971).
Noble-Nesbitt, J.: Water balance in the firebrat Thermobia domestica (Packard). Exchanges of water from the atmosphere. J. exp. Biol. 50, 745–769 (1969).
Noble-Nesbitt, J.: Water balance in the firebrat Thermobia domestica (Packard). The site of uptake of water from the atmosphere. J. exp. Biol. 52, 193–200 (1970).
Noirot, C., Noirot-Timothée, C.: Revêtement de la membrane cytoplasmique et absorption des ions dans les papilles rectales d'un Termite (Insecta, Isoptera). C. R. Acad. Sci. (Paris) 263, 1099–1102 (1966).
Noirot, C., Noirot-Timothée, C.: L'epithélium absorbant de la panse d'un termite supérieur. Ultrastructures et rapport avec la symbiose bactérienne. Ann. Soc. ent. Fr. 3, 577–592 (1967).
Noirot, C., Noirot-Timothée, C.: Ultrastructure du proctodeum chez Thysanoure Lepismodes inquilinus Newman (= Thermobia domestica Packard). II Le sac anal. J. Ultrastruct. Res. 37, 335–350 (1971).
Oschman, J. L., Wall, B. J.: The structure of the rectal pads of Periplaneta americana L. with regard to fluid transport. J. Morph. 127, 475–510 (1969).
Pease, D. C.: Histological techniques for electron microscopy, sec. ed. New York-London: Academic Press 1964.
Phillips, J. E.: Apparent transport of water by insect excretory systems. Amer. Zool. 10, 413–436 (1970).
Phillips, J. E., Meredith, J.: Active sodium and chloride transport by anal papillae of a salt water mosquito larvae (Aedes campestris). Nature (Lond.) 222, 168–169 (1969).
Prosser, C. L., Brown, F. A.: Comparative animal physiology. Philadelphia-London 1950.
Ramsay, J. A.: Osmotic regulation in mosquito larvae. J. exp. Biol. 27, 145–157 (1950).
Reynolds, E. S.: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963).
Sabatini, D. D., Bensch, K., Barrnett, R. J.: Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 17, 19–58 (1963).
Schmidt-Nielsen, B.: Introduction to comparative aspects of transport of hypertonic, isotonic, and hypotonic solutions by epithelial membranes: Physiology Society Symposium. Fed. Proc. 30, 3–5 (1971).
Shaw, J., Stobbart, R. H.: Osmotic and ionic regulation in insects. Advanc. Insect Physiol. 1, 315–399 (1963).
Stobbart, R. H., Shaw, J.: Salt and water balance: excretion. In: Rockstein, M. (ed.), The physiology of insecta, vol. 3, p. 189–258. New York-London: Academic Press 1964.
Wall, B. J.: Local osmotic gradients in the rectal pads of an insect. Fed. Proc. 30, 42–48 (1971).
Wessing, A.: Funktionsmorphologie von Exkretionsorganen bei Insekten. Zool. Anz., Suppl. 31, 633–681 (1967).
Witkus, E. R., Grillo, R. S., Smith, W. J.: Microtubules of the hindgut epithelium in the woodlouse, Oniscus ascellus. Proc. E.M. Soc. Amer. 27, 312–313 (1969).
Author information
Authors and Affiliations
Additional information
This work was supported by operating grants from the National Research Council of Canada.
Rights and permissions
About this article
Cite this article
Meredith, J., Phillips, J.E. Rectal ultrastructure in salt- and freshwater mosquito larvae in relation to physiological state. Z.Zellforsch 138, 1–22 (1973). https://doi.org/10.1007/BF00307074
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00307074