Abstract
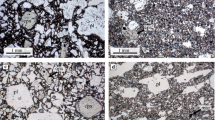
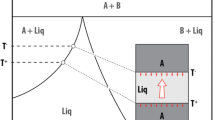
A large (4.8 m3, 1.3x107 g) artificial mafic melt with a bulk composition similar to that of a basalt (but with a high CaO content of 17 wt%) was generated during a demonstration of in situ vitrification and was allowed to cool naturally. During the melting process, convection was vigorous, resulting in a chemically and thermally homogeneous melt body. Once heating was complete, the cooling rate was rapid with the temperature dropping from 1500°C to 500°C in ∼6 days within the interior of the 3 m diameter, 1.5 m thick body. A ∼20 h period of constant temperature (1140°C) observed during colling was the result of latent heat released by widespread crystallization. The final crystalline assemblage consists of diopsidic to hedenbergitic pyroxene and anorthitic feldspar, with a subordinate amount of potassic feldspar, plus a small amount of evolved glass. The compositions and proportions of phases agree well with those predicted by the MELTS thermodynamic model. Thermal and textural evidence suggest that convection within the melt ceased coincident with formation of the first crystals. Textural investigation of core samples reveals large (up to 1 cm in length) acicular diopsidic pyroxenes in a matrix of smaller feldspar and zoned pyroxene crystals (∼500 μm in length). Crystal shape and pyroxene composition vary as a function of position within the solidified body, as a function of cooling rate. Both crystal size and degree of crystallinity are highest in the central, most slowly-cooled parts of the rock. Crystal shape is characterized by tabular, equilibrium-growth forms in the slowly-cooled areas, grading to highly skeletal, dendritic forms at the rapidly-cooled edges of the body. The pyroxene crystals are dominantly homogeneous diopside, but crystals are characterized by thin Fe-rich hedenbergitic rims. These rims were deposited when Mg in the melt was depleted by diopside growth, and melt temperature had cooled sufficiently to allow Fe-rich pyroxene growth. Crystal growth rates can be calculated based on thermal behavior of the melt, reinforced by thermodynamic modelling, and are determined to be between 10-7 and 10-8 cm/s in the central part of the melt. These estimates agree well with growth rates in natural systems with similar cooling rates. Pyroxene crystals that formed at a higher cooling rates are characterized by higher Al and lower Mg contents relative to tabular equilibrium crystalline forms, presumably as a result of disequilibrium melt compositions at the crystal-melt interface.
Similar content being viewed by others
References
Albarede F, Bottinga Y (1972) Kinetic disequilibrium in trace element paritioning between phenocrysts and host lava. Geochim Cosmochim Acta 36: 141–156
Alexiades V, Jacobs GK (1994) Solidification modelling of In Situ Vitrification melts. In: Ladde GS, Sambandham M (eds) Proceedings of Dynamic Systems and Applications Vol. 1 Dynamic Publishers, Atlanta, pp 11–16
Bence AE, Albee AL (1968) Empirical correction factors for the electron microanalysis of silicates and oxides. J Geol 76: 382–403
Bence AE, Papike JJ, Prewitt CT (1970) Apollo 12 clinopyroxenes: chemical trends. Earth Planet Sci Lett 8: 393–399
Brandeis G, Marsh BD (1989) The convective liquidus in a solidifying magma chamber: a fluid dynamic investigation. Nature 339: 613–616
Brandeis G, Jaupart C, Allegre CJ (1984) Nucleation, crystal growth and the thermal regime of cooling magmas. J Geophys Res 89: 10161–10177
Buelt JL, Timmerman CL, Oma KH, Fitzpatrick VF, Carter JG (1987) In Situ Vitrification of transuranic wastes: an updated systems evaluation and applications assessment. PNL Internal Rep 4800 (Pacific Northwest Laboratory, Richland, Washington)
Burton JA, Prim RC, Slichter WP (1953) The distribution of solute in crystals growth from the melt, part 1. Theoretical. J Chem Phys 21: 1987–1991
Cashman KV (1992) Groundmass crystallization of Mount St. Helens dacite, 1980–1986: a tool for interpreting shallow magmatic processes. Contrib Mineral Petrol 109: 431–449
Cashman KV (1993) Relationship between plagioclase crystallization and cooling rate in basaltic melts. Contrib Mineral Petrol 113: 126–142
Cashman KV, Marsh BD (1988) Crystal size distributions (CSD) in rocks and the kinetics and dynamics of crystallization II. Makaopuhi lava lake. Contrib Mineral Petrol 99: 292–305
Deer WA, Howie RA, Zussman J (1994) An introduction to the rock forming minerals. Longman, London
Dowty E (1980) Crystal growth and nucleation theory and the numerical simulation of igneous crystallization. In: Hargraves RB (ed) Physics of magmatic processes. Princeton University Press, Princeton, N.J.
Dunbar NW, Riciputi LR, Jacobs GK, Naney MT (1993) Generation of rhyolitic melt in an artificial magma: implications for fractional crystallization process in natural magmas. J Volcanol Geotherm Res 57: 157–166
Dunbar NW, Cashman KV, Dupre R (1994) Crystallization processes of anorthoclase phenocrysts in the Mount Erebus magmatic system: evidence from crystal composition, crystal size distributions and volatile contents of melt inclusions. In: Kyle PR (ed) Volcanological studies of Mount Erebus, 1st edn. American Geophysical Union, Vol 66, Washington DC, p 129–146
Gamble RP, Taylor LA (1980)_Crystal/liquid partitioning in augite: effects of cooling rate. Earth Plannet Sci Lett 47: 21–33
Ghiorso MS (1987) Modelling magmatic systemst thermodynamic relations. In: Carmichael ISE, Eugster HP (eds) Thermodynamic modelling of geological materials: minerals, melts and fluids (Reviews in Mineralogy, vol. 17). Mineralogical Society of America, Washington DC, pp 443–465
Ghiroso MS, Carmichael ISE (1987) Modelling magmatic systems: petrological applications. In: Carmichael ISE, Eugster HP (eds) Thermodynamic modelling of geological materials: minerals, melts and fluids (Reviews in Mineralogy, vol. 17). Mineralogical Society of America, Washington DC, pp 467–499
Grove TL, Bence AE (1977) Experimental study of pyroxene-liquid interaction in quartz-normative basalt. Proc 8th Lunar Sci Conf: 1549–1579
Hort M, Spohn T (1991) Numerical simulation of the crystallization of multicomponent melts in the thin dikes or sills 2. Effects of heterocatalytic nucleation and composition. J Geophys Res 96: 485–499
Jacobs GK, Dunbar NW, Naney MT, Williams RT (1992) In Situ Vitrification: observations of petrological processes in a man-mande magmatic system. EOS, Trans Am Geophys Union 73: 401–411
Jacobs GK, Naney MT, Dunbar NW (1993) Evidence of convection in a large, artificial magma: thermal oscillations and cooling rates recorded during crystallization. Geol Soc Am Abstr Prog 25: A367
Jacobs JW, Korotev RL, Blanchard DP, Haskins LA (1977) A well-tested procedure for instrumental neutron-activation analysis of silicate rocks and minerals. Geochim Cosmochim Acta 40: 93–114
Kirkpatrick RJ (1976) Towards a kinetic model for the crystallization of magma bodies. J Geophys Res 81: 2565–2571
Korotev RL, Lindstrom DJ (1985) Inteferences from fission of U-235 in INAA of rocks. Trans Am Nucl Soc 49: 177–178
Kyle PR (1977) Mineralogy and glass chemistry of recent volcanic ejecta from Mt Erebus, Ross Island, Antarctica. New Zealand J Geol Geophys 20: 1123–1146
Lange RA, Cashman KV, Navrotsky A (1991) Direct measurements of the distribution of latent heat during cyrstallization and melting of a ugandite and an olivine basalt. Geol Soc Am Abstr Prog 23: A93
Lindsley DH (1983) Pyroxene geothermometry. Am Mineral 68: 477–493
Lofgren GE (1980) Experimental studies on the dynamic crystallization of silicate melts. In: Hargrave RB (ed) Physics of magmatic processes. Princeton University Press, Princeton, N.J.
Lofgren GE (1983) Effect of heterogenous nucleation of basaltic textures: a dynamic crystallization study. J Petrol 24: 229–255
Norish K, Chappell BW (1977) X-ray fluorescence spectrometry. In: Zussman J (ed) Physical methods in determinative mineralogy. Academic Press, San Diego, Calif.
Ryan MP, Blevins JYK (1987) The viscosity of synthetic and natural silicate melts and glasses at high temperatures and 1 bar (105 Pascals) pressure and higher pressures. US Geol Surv Bull 1764: 1–563
Shaw HR (1972) Viscosity of magmatic silicate liquids: an empirical method of prediction. Am J Sci 272: 870–893
Swanson SE, Fenn PM (1986) Quartz crystallization in igneous rocks. Am Mineral 71: 331–342
Winkler HGF (1947) Kristallgrosse und Abkuhlung. Heidelb Beitr Mineral Petrogr 1: 87–104
Author information
Authors and Affiliations
Additional information
New Mexico Bureau of Mines and Mineral Resources, New Mexico Institute of Mining and Technology, Socorro, NM 87801, USA
Rights and permissions
About this article
Cite this article
Dunbar, N.W., Jacobs, G.K. & Naney, M.T. Crystallization processes in an artificial magma: variations in crystal shape, growth rate and composition with melt cooling history. Contr. Mineral. and Petrol. 120, 412–425 (1995). https://doi.org/10.1007/BF00306518
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00306518