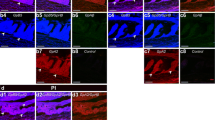
Abstract
To better understand neuroendocrine regulation and the intracellular mechanisms mediating pituitary-hormone release, it is necessary to study the physiology of identified single cells. We have developed a system to identify gonadotropin, growth-hormone, and prolactin cells in primary cultures of goldfish pituitary cells. Using Nomarski differential interference-contrast microscopy, the unique morphologies of discrete subpopulations of cells were characterized. To aid in the initial characterization of different pituitary-cell types, a discontinuous Percoll density-gradient cell-separation technique was developed. This method provided fractions enriched with functional gonadotropin, growth-hormone, and prolactin cells. The morphology of each cell type was initially characterized in enriched fractions of immunofluorescently labelled cells using differential interference-contrast microscopy. The cell type-specific morphologies were then confirmed in live pituitary-cell cultures. Gonadotropin, growth-hormone, and prolactin cells were correctly identified in live pituitary-cell cultures. Gonadotropin, growth-hormone, and prolactin cells were correctly identified in live mixed cultures in 92, 94, and 100% of the trials, respectively. The ability to directly identify cells in primary cultures allows the physiological study of identified single cells with minimal pretreatment.
Similar content being viewed by others
References
Chang JP, Leeuw R de (1990) In vitro goldfish growth hormone responses to gonadotropin-releasing hormone: possible roles of extracellular calcium and arachidonic acid metabolism? Gen Comp Endocrinol 80:155–164
Chang JP, Freedman GL, Leeuw R de (1989) Participation of arachidonic acid metabolism in gonadotropin-releasing hormone stimulation of goldfish gonadotropin release. Gen Comp Endocrinol 76:2–11
Chang JP, Cook H, Freedman GL, Wiggs AJ, Somoza GM, Leeuw R de, Peter RE (1990 a) Use of a pituitary cell dispersion method and primary culture system for the studies of gonadotropin-releasing hormone action in the goldfish, Carassius auratus. I. Initial morphological, static, and cell column perifusion studies. Gen Comp Endocrinol 77:256–273
Chang JP, Yu KL, Wong AOL, Peter RE (1990 b) Differential actions of dopamine receptor subtypes on gonadotropin and growth hormone release in vitro in goldfish. Neuroendocrinology 51:664–674
Chang JP, Wildman B, van Goor F (1991) Lack of involvement of arachidonic acid metabolism in chicken gonadotropin-releasing hormone II (cGnRH-II) stimulation of gonadotropin secretion in dispersed pituitary cells of goldfish, Carassius auratus. Identification of a major difference in salmon GnRH and chicken GnRH-II mechanisms of action. Mol Cell Endocrinol 79:75–83
Chang JP, Jobin RM, Wong AOL (1993) Intracellular mechanisms mediating gonadotropin and growth hormone release in the goldfish, Carassius auratus. Fish Physiol Biochem 11:25–33
Childs D, Hyde C, Naor Z (1983) Morphometric analysis of thyrotropes in developing and cycling female rats: studies of intact pituitaries and cell fractions separated by centrifugal elutriation. Endocrinology 113:1601–1607
Cook H, Berkenbosch JW, Femhout MJ, Yu KL, Peter RE, Chang JP, Rivier JE (1991) Demonstration of gonadotropin releasing-hormone receptors on gonadotrophs and somatotrophs of the goldfish: an electron microscope study. Regul Pept 36:369–378
Denef C, Andries M (1983) Evidence for paracrine interaction between gonadotrophs and lactotrophs in pituitary cell aggregates. Endocrinology 112:813–822
Habibi HR, Peter RE, Sokolowska M, Rivier JE, Vale WW (1987) Characterization of gonadotropin-releasing hormone binding to pituitary receptors in goldfish. Biol Reprod 4:844–853
Habibi HR, Leeuw R de, Nahorniak CS, Goos HJT, Peter RE (1989) Pituitary gonadotropin-releasing hormone (GnRH) receptor activity in goldfish and catfish: seasonal and gonadal effects. Fish Physiol Biochem 7:109–118
Hymer WC, Hatfield JM (1984) Separation of cells form the rat anterior pituitary gland. In: Pretlowll T, Pretlowll TP (eds) Cell separation methods and selected applications. Academic Press, New York, pp 163–194
Jobin RM, Chang JP (1992 a) Differences in extracellular calcium involvement mediating the secretion of gonadotropin and growth hormone stimulated by two closely related endogenous GnRH peptides in goldfish pituitary cells. Neuroendocrinology 55:156–166
Jobin RM, Chang JP (1992 b) Actions of two native GnRHs and protein kinase C modulators on goldfish pituitary cells. Studies on intracellular calcium levels and gonadotropin release. Cell Calcium 13:531–540
Kaul S, Vollrath L (1974) The goldfish pituitary. I. Cytology. Cell Tissue Res 154:211–230
Leeuw R de, Goos HJT, Peute J, Pelt AMM van, Burzawa-Gerard E, Oordt PGWJ van (1984) Isolation of gonadotropes from the pituitary of the African catfish, Clarias lazera. Morphological and physiological characterization of purified cells. Cell Tissue Res 236:669–675
Luque EH, Toro MM de, Smith PF, Neill JD (1986) Subpopulations of lactotropes detected with the reverse hemolytic plaque assay show differential responsiveness to dopamine. Endocrinology 118:2120–2124
Marchant TA, Dulka JG, Peter RE (1989) Relationship between serum growth hormone levels and the brain and pituitary content of immunoreactive somatostatin in the goldfish, Carassius auratus. Gen Comp Endocrinol 73:458–468
Nagahama Y (1973) Histo-physiological studies on the pituitary gland of some teleost fishes, with special reference to the classification of hormone-producing cells in the adenohypophysis. Mem Fac Fish Hokkaido Univ 21:1–63
Peter RE, Nahorniak CS, Chang JP, Crim LW (1984) Gonadotropin release from the pars distalis of goldfish, Carassius auratus, transplanted beside the brain or into brain ventricle: additional evidence for gonadotropin-release-inhibitory factor. Gen Comp Endocrinol 55:337–346
Peter RE, Chang JP, Nahorniak CS, Omeljaniuk RJ, Sokolowska M, Shih SH, Billard R (1986) Interactions of catecholamines and GnRH in regulation of gonadotropin secretion in teleost fish. Recent Prog Horm Res 42:513–547
Peter RE, Yu KL, Marchant TA, Rosenblum PM (1990 a) Direct neural regulation of the teleost adenohypophysis. J Exp Zool 4[Suppl]:84–89
Peter RE, Habibi HR, Chang JP, Nahorniak CS, Yu KL, Huang YP, Marchant TA (1990 b) Actions of gonadotropin releasing hormone (GnRH) in the goldfish. In: Epple A, Scanes CG, Stetson MH (eds) Progress in comparative endocrinology. Wiley-Liss, New York, pp 393–398
Sage M, Bern HA (1971) Cytophysiology of the teleost pituitary. Int Rev Cytol 31:339–375
Sokolowska M, Peter RE, Nahomiak CS, Chang JP (1985) Seasonal effects of pimozide and des Gly10[D-Ala6]LHRH ethylamide on gonadotropin secretion in goldfish. Gen Comp Endocrinol 57:472–479
Tse A, Hille B (1992) GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science 255:462–464
Van Der Kraak G, Suzuki K, Peter RE, Itoh H, Kawauchi H (1992) Properties of common carp gonadotropin I and gonadotropin II. Gen Comp Endocrinol 85:217–229
Wong AOL, Chang JP, Peter RE (1992) Dopamine stimulates growth hormone release from the pituitary of goldfish, Carassius auratus, through the dopamine D1 receptor. Endocrinology 130:1201–1210
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Goor, F., Goldberg, J.I., Wong, A.O.L. et al. Morphological identification of live gonadotropin, growth-hormone, and prolactin cells in goldfish (Carassius auratus) pituitary-cell cultures. Cell Tissue Res 276, 253–261 (1994). https://doi.org/10.1007/BF00306111
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00306111