Abstract
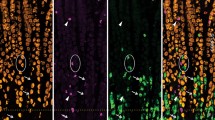
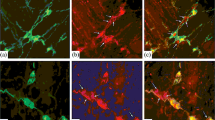
The expression of the gene for vasoactive intestinal polypeptide (VIP) and peptide histidine methionine (PHM) in the human gastrointestinal tract was studied by in situ hybridization and Northern blotting for PHM/ VIP mRNA and immunocytochemistry using specific antisera against the bioactive peptides PHM and VIP. In the colon sigmoideum, antisera against all five putative processing products of the VIP precursor (prepro-VIP) were used, namely prepro-VIP 22–79, PHM, prepro-VIP 111–122, VIP and prepro-VIP 156–170. Furthermore, RNA extracted from various regions of the gastrointestinal tract was examined by Northern blots and hybridization to a VIP-cDNA probe. Throughout the gastrointestinal tract, PHM/VIP mRNA was found in neurons only. Using single-or double-staining methods, we demonstrated both PHM/VIP mRNA and the corresponding peptides PHM and VIP in the neurons. In the sigmoideum, the single-staining methods were extended to investigate whether the neurons simultaneously contained PHM/VIP mRNA and each of the five prepro-VIP-derived peptides. Only one major band of PHM/VIP mRNA (1.9 kb) was found by Northern blotting in the tissue of the gastrointestinal tract.
Similar content being viewed by others
References
Amara SG, Jonas V, Rosenfeld MG (1982) Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298:240–244
Bloch B, Guitteny AF, Normand E, Chouham S (1990) Presence of neuropeptide messenger RNAs in neuronal processes. Neurosci Lett 109:259–264
Bodanszky M, Klausner YS, Lin CY, Mutt V, Said SI (1974) Synthesis of the vasoactive intestinal peptide (VIP). J Am Chem Soc 96:4973–4978
Bredkjær HE, Rønnoy-Jessen D, Fahrenkrug L, Ekblad E, Fahrenkrug J (1991) Expression of prepro VIP derived peptides in the human gastrointestinal tract: a biochemical and immunocytochemical study. Regul Pept 33:145–164
Chew L-J, Murphy D, Carter DA (1991) Differential use of 3′poly(A) addition sites in vasoactive intestinal peptide messenger ribonucleic acid of the rat anterior pituitary gland. J Neuroendocrinol 3:351–355
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Couwenhoven RI, Luo W, Snead ML (1990) Co-localization of EGF transcripts and peptides by combined immunocytochemistry and in situ hybridization. J Histochem Cytochem 38:1853–1857
Dirks RW, Raap AK, Minnen J van, Vreugdenhil E, Smit AB, Ploeg M van der (1989) Detection of mRNA molecules coding for neuropeptide hormones of the pond snail Lymnaea stagnalis by radioactive and non-radioactive in situ hybridization: a model for mRNA detection. J Histochem Cytochem 37:7–14
Fahrenkrug J (1989) VIP and the autonomic neurotransmission. Pharmacol Ther 41:515–534
Fahrenkrug J, Emson PC (1989) Characterization and regional distribution of peptides derived from the VIP precursor in the normal human brain. J Neurochem 53:1142–1148
Gozes I, Bodner M, Scani Y, Fridkin M (1984) Detection of mRNAs containing regulatory peptide coding sequences using synthetic oligodeoxynucleotides. J Cell Biochem 26:147–156
Gozes I, Giladi E, Shani Y (1987) Vasoactive intestinal peptide gene: putative mechanism of information storage of the RNA level. J Neurochem 48:1136–1141
Hökfelt T, Schultzberg M, Lundberg JM, Fuxe K, Mutt V, Fahrenkrug J, Said SI (1982) Distribution of vasoactive intestinal polypeptide in the central and peripheral nervous systems as revealed by immunocytochemistry. In: Said ST (ed) Vasoactive intestinal peptide. Raven Press, New York, pp 65–90
Itoh N, Okaba K, Yanaihara N, Okamoto H (1983) Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature 304:547–549
Kiyama H, Emson PC, Ruth J (1990 a) Distribution of tyrosin hydroxylase mRNA in the rat central nervous system visualized by alkaline phosphatase in situ hybridization histochemistry. Eur J Neurosci 2:512–524
Kiyama H, Emson PC, Ruth J, Morgan C (1990b) Sensitive non-radioisotopic in situ hybridization histochemistry: demonstration of tyrosine hydroxylase gene expression in rat brain and adrenal. Mol Brain Res 7:213–219
Larsson L-I, Hougaard DM (1991) Combined non-radioactive detection of peptide hormones and their mRNAs in endocrine cells. Histochemistry 96:375–380
Larsson L-I, Fahrenkrug J, Schaffalitzky de Muckadell O, Sundler F, Håkanson R, Rehfeld JF (1976) Localization of vasoactive intestinal peptide (VIP) to the central and peripheral neurons. Proc Natl Acad Sci USA 73:3197–3200
Larsson L-I, Polak JM, Buffa R, Sundler F, Solcia E (1979) On the immunocytochemical localization of the vasoactive intestinal polypeptide. J Histochem Cytochem 27:936–938
Lewis ME, Krause RG, Roberts-Lewis JM (1988) Recent developments in the use of synthetic oligonucleotides for in situ hybridization histochemistry. Synapse 2:308–316
Linder S, Barkheim T, Norberg A, Persson H, Schalling M, Hökfelt T, Magnusson G (1987) Structure and expression of the gene encoding the vasoactive intestinal peptide precursor. Proc Natl Acad Sci USA 84:605–609
Mutt V, Said SI (1974) Structure of the porcine vasoactive intestinal octacosapeptide. Eur J Biochem 42:581–589
Nishizawa M, Hayakawa Y, Yanaihara N, Okamoto H (1985) Nucleotide sequence divergence and functional constraint in VIP precursor mRNA evolution between human and rat. FEBS Lett 183:55–59
Polak JM, Pearse AGE, Garaud J-C, Bloom SR (1974) Cellular localization of a vasoactive intestinal peptide in the mammalian and avian gastrointestinal tract. Gut 15:720–724
Rosenfeld MG, Mermod JJ, Amara SG, Swanso LW, Sawchenko PE, Rivier J, Vale WW, Evan RM (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304:129–135
Ruth J, Morgan C, Pasko A (1985) Linker arm nucleotide analogs useful in oligonucleotide synthesis. DNA Cell Biol 4:93
Said SI, Mutt V (1970a) Polypeptide with broad biological activity: isolation from small intestine. Science 169:1217–1218
Said SI, Mutt V (1970b) Potent peripheral and splanchnic-vasodilator peptide from normal gut. Nature 225:863–864
Schalling M, Hökfelt T, Wallace B, Goldstein M, Filer D, Yamin C, Schlesinger DH (1986) Tyrosine 3-hydroxylase in rat brain and adrenal medulla: hybridization, histochemistry and immunocytochemistry combined with retrograde tracing. Proc Natl Acad Sci USA 83:6208–6212
Shivers BD, Harlan RE, Pfaff DW, Schacter BS (1986) Combination of immunocytochemistry and in situ hybridization in the same tissue section of rat pituitary. J Histochem Cytochem 34:39–43
Sternberger LA, Hardy PH, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry. J Histochem Cytochem 18:315–333
Tsukada T, Horovitch SJ, Montminy MR, Mandel G, Goodman RH (1985) Structure of the human vasoactive intestinal polypeptide gene. DNA Cell Biol 4:293–300
Yamagami T, Ohsawa K, Nishizawa M, Inoue C, Gotoh E, Yanaihara N, Yamamoto H, Okamoto H (1988) Complete nucleotide sequence of human vasoactive intestinal peptide/PHM-27 gene and its inducible promotor. Vasoactive intestinal peptide and related peptides. Ann NY Acad Sci 527:87–101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bredkjær, H.E., Wulff, B.S., Emson, P.C. et al. Location of PHM/VIP mRNA in human gastrointestinal tract detected by in situ hybridization. Cell Tissue Res 276, 229–238 (1994). https://doi.org/10.1007/BF00306108
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00306108