Abstract
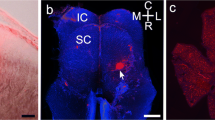
Presumed dopaminergic neurons were visualized in the retina of the clawed frog, Xenopus laevis, by anti-tyrosine hydroxylase (TH) immunoreactivity. The studied cells constitute a uniform population with perikarya at the junction of inner nuclear (INL) and inner plexiform (IPL) layers. Each cell body gives rise to 4–6 relatively stout processes (0.5–2.0 μm in diameter) which run for up to 1.2 mm in strata 4–5 of the IPL. These processes have a very asymmetric distribution in the horizontal plane of the retina. A dense plexus of TH fine fibers is distributed uniformly in stratum 1 of the IPL. TH cells are distributed evenly but sparsely (16–20 cells/mm2) across the retina. About 20% of the TH neurons emit 1–3 distally directed fine processes, the majority of which extend < 20 μm, which barely suffices to reach the outer plexiform layer (OPL). Other longer processes are typically unbranched; some reach the OPL, others run tangentially in the INL. The axon terminals of Golgi-impregnated bipolar cells are characterized according to the strata of the IPL in which they arborize. About 80% are confined either to strata 1–2 or 3–5, conforming to the ‘off’ and ‘on’ zones defined by Famiglietti and Kolb (1976). The remainder appear to end in both zones, some extending across the entire width of the IPL. EM examination showed that TH processes receive bipolar synaptic input in both distal and proximal portions of the IPL.
Similar content being viewed by others
References
Besharse JC (1992) The “on”-bipolar agonist, L-2-amino-4-phosphonobutyrate, blocks light-evoked cone contraction in Xenopus eye cups. Neurochem Res 17:75–80
Besharse JC, Witkovsky P (1992) Light-evoked contraction of red absorbing cones in the Xenopus retina is maximally sensitive to green light. Vis Neurosci 8:243–249
Boatright JH, Hoel MJ, Iuvone PM (1989) Stimulation of endogenous dopamine release and metabolism in amphibian retina by light-and K+. Brain Res 482:164–168
Boycott BB, Wassle H (1974) The morphological types of ganglion cells of the dometic cat's retina. J Physiol 240:397–419
Brecha NC, Oyster CW, Takahashi ES (1984) Identification and characterization of tyrosine hydroxylase-immunoreactive amacrine cell. Invest Ophthal Vis Sci 25:66–70
Cajal RS y (1892) La rétine des vertébrés. La Cellule 9:121–246
Critz SD, Mare RE (1992) Glutamate antagonists that block hyperpolarizing bipolar cells increase the release of dopamine from turtle retina. Vis Neurosci 9:271–278
Dearry A, Edelman J, Miller S, Burnside B (1990) Dopamine induces light-adaptive retinomotor movements in bullfrog cones via D2 receptors and in retinal pigment epithelium via D1 receptors. J Neurochem 54:1367–1378
Djamgoz MBA, Wagner H-J (1991) Localization and function of dopamine in the adult vertebrate retina. Neurochem Int 20:139–191
Dong C-J, McReynolds JS (1991) The relationship between light, dopamine release and horizontal cell coupling in the mudpuppy retina. J Physiol 440:291–309
Dowling JE, Ehinger B (1975) Synaptic organization of the aminecontaining interplexiform cells of the goldfish and Cebus monkey retina. Science 188:270–273
Dowling JE, Ehinger B (1978) The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc Trans R Soc Lond [Biol] 201:7–26
Ehinger B (1982) Neurotransmitter systems in the retina. Retina 2:305–321
Ehinger B, Falck B, Laties AM (1969) Adrenergic neurons in teleost retina. Z Zellforsch Mikrosk Anat 97:285–297
Famiglietti EV, Kolb H (1976) Structural basis of ON-and OFF-center responses in retinal ganglion cells. Science 194:193–195
Gabriel R, Zhu B, Straznicky C (1991) Tyrosine hydroxylase-immunoreactive elements in the distal retina of Bufo marinus: light and electron miocroscope study. Brain Res 559:225–232
Greferath U, Grunert U, Wassle H (1990) Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol 301:433–442
Haggendal J, Malmfors T (1965) Identification and cellular localization of the catecholamines in the retina and the choroid of the rabbit. Acta Physiol Scand 64:58–66
Hanani M, Vallerga S (1980) Rod and cone signals in the horizontal cells of the tiger salamander retina. J Physiol 298:397–405
Hare WA, Owen WG (1993) Dopamine uncouples rod photoreceptors in the tiger salamander retina. Invest Ophthal Vis Sci 34:753
Hare WA, Lowe JS, Owen G (1986) Morphology of physiologically identified bipolar cells in the retina of the tiger salamander, Ambystoma tigrinum. J Comp Neurol 252:130–138
Hokoc JN, Mariani AP (1988) Synapses from bipolar cells onto dopaminergic amacrine cells in cat and rabbit retinas. Brain Res 461:17–26
Kolb H, Cline C, Wang HH, Brecha N (1987) Distribution and morphology of dopaminergic amacrine cells in the retina of the turtle (Pseudemys scripta elegans). J Neurocytol 16:577–588
Kolb H, Cuenca N, Wang H-H, Dekorver L (1990) The synaptic organization of the dopaminergic amacrine cell in the cat retina. J Neurocytol 19:343–366
Lasansky A (1973) Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond [Biol] 265:471–489
Muresan Z, Besharse JC (1993) D2-like dopamine receptors in amphibian retina: localization with fluorescent ligands. J Comp Neurol 331:149–160
Negishi K, Kato S, Teranishi T (1988) Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett 108:279–283
Nguyen-Legros J, Versaux-Botteri C, Vigny A, Raoux N (1985) Tyrosine hydroxylase immunohistochemistry fails to demonstrate dopaminergic interplexiform cells in the turtle retina. Brain Res 339:323–328
Nguyen-Legros J, Moussafi F, Simon A (1990) Sclerally directed processes of dopaminergic interplexiform cells reach the outer nuclear layer in rat and monkey retina. Vis Neurosci 4:547–553
Oyster CW, Takahashi ES, Cilluffo M, Brecha NM (1985) Morphology and distribution of tyrosine hydroxylase-like immunoreactive neurons in the cat rétina. Proc Natl Acad Sci USA 82:6335–6339
Piccolino M, Neyton J, Gerschenfeld HM (1984) Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′:5′-monophosphate in horizontal cells of turtle retina. J Neurosci 4:2477–2488
Pierce ME, Besharse JC (1985) Circadian regulation of retinomotor movements I. Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol 86:671–689
Pollard J, Eldred WD (1990) Synaptic analysis of amacrine cells in the turtle retina which contain tyrosine hydroxylase-like immunoreactivity. J Neurocytol 19:53–66
Schutte M, Witkovsky P (1990) Serotonin-like immunoreactivity in the retina of the clawed frog Xenopus laevis. J Neurocytol 19:504–518
Schutte M, Witkovsky P (1991) Dopaminergic interplexiform cells and centrifugal fibers in the Xenopus retina. J Neurocytol 20:195–207
Slaughter MM, Miller RF (1981) 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retinal research. Science 211:182–185
Stell WK, Witkovsky P (1973) Retinal structure in the smooth dogfish, Mustelus canis: general description and light microscopy of giant ganglion cells. J Comp Neurol 148:1–32
Stone S, Schutte M (1991) Physiological and morphological properties of OFF-and ON-center bipolar cells in the Xenopus retina: effects of glycine and GABA. Vis Neurosci 7:363–376
Tork I, Stone J (1979) Morphology of catecholamine-containing amacrine cells in the cat's retina, as seen in retinal whole mounts. Brain Res 169:261–273
Van Haesendonck E, Marc RE, Missotten L (1993) New aspects of dopaminergic interplexiform cell organization in the goldfish retina. J Comp Neurol 333:503–518
Voigt T, Wassle H (1987) Dopaminergic innervation of AII amacrine cells in mammalian retina. J Neurosci 7:4115–4128
Wagner H-J, Behrens UD (1993) Microanatomy of the dopaminergic system in the rainbow trout retina. Vision Res 33:1345–1358
Watt CB, Glazebrook PA (1993) Synaptic organization of dopaminergic amacrine cells in the larval tiger salamander retina. Neuro-science 53:527–536
Watt CB, Yang SZ, Lam DMK, Wu SM (1988) Localization of tyrosine hydroxylase-like immunoreactive amacrine cells in the larval tiger salamander retina. J Comp Neurol 272:114–126
Witkovsky P, Dearry A (1991) Functional roles of dopamine in the vertebrate retina. Progr Ret Res 11:247–292
Witkovsky P, Eldred W, Karten HJ (1984) Catecholamine and indoleamine-containing neurons in the turtle retina. J Comp Neurol 228:247–255
Witkovsky P, Stone S, MacDonald ED (1988) Morphology and synaptic connections of HRP-filled, axon-bearing horizontal cells in the Xenopus retina. J Comp Neurol 275:29–38
Witkovsky P, Nicholson C, Rice ME, Bohmaker K, Meller E (1993) Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proc Natl Acad Sci USA 90:5667–5771
Yazulla S, Zucker CL (1988) Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis Neurosci 1:13–29
Zhu B, Straznicky C (1990) Dendritic morphology and retinal distribution of tyrosine hydroxylase-like immunoreactive amacrine cells in Bufo marinus. Anat Embryol 181:365–371
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Witkovsky, P., Zhang, J. & Blam, O. Dopaminergic neurons in the retina of Xenopus laevis: amacrine vs. interplexiform subtypes and relation to bipolar cells. Cell Tissue Res 278, 45–56 (1994). https://doi.org/10.1007/BF00305777
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305777