Summary
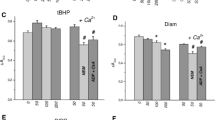
In intact tissue sections, neotetrazolium formazan production is greater when the mitochondrial succinate dehydrogenase and the cytoplasmic glucose-6-phosphate dehydrogenase activities are tested together than when measured individually. Studies with malonate have shown that this increase is due to an increase in the rate of oxidation of succinate. Such an increase does not occur however when the hydrogen transport pathway is by-passed with PMS. It is suggested that the cytoplasmic activity overcomes a rate-limiting step in the mitochondrial transport chain.
Similar content being viewed by others
References
Altman, F. P.: The cellular chemistry of certain cytoplasmic oxidative enzymes, and their relevance to the metabolism of cancers. PhD. Thesis, Univ. of London. 1968.
Altman, F. P.: The use of eight different tetrazolium salts for a quantitative study of pentose shunt dehydrogenation. Histochemie 19, 363–374 (1969).
Altman, F. P., Chayen, J.: The retention of nitrogenous material in unfixed sections during incubation for histochemical demonstration of enzymes. Nature (Lond.) 207, 1205–1206 (1965).
Altman, F. P., Chayen, J.: The significance of a functioning hydrogen-transport system for the retention of ‘soluble’ dehydrogenases in unfixed sections. J. roy. micr. Soc. 85, 175–180 (1966).
Bendall, D. S., de Duve, D.: Tissue fractionation studies. 14. The activation of latent dehydrogenases in mitochondria from rat liver. Biochem. J. 74, 444–450 (1960).
Butcher, R. G.: Studies on succinate oxidation: I. The use of intact tissue sections. Exp. Cell Res. 60, 54–60 (1970).
Butcher, R. G., Altman, F. P., Chayen, J.: The significance of the gas phase for dehydrogenase histochemistry. Proc. roy. micr. Soc. 1, 215 (1966).
Butcher, R. G., Chayen, J.: The value of intact tissue sections for studying metabolic interactions between the cytoplasm and mitochondria. Exp. Cell Res. 49, 656–665 (1968).
Chayen, J., Bitensky, L., Butcher, R. G., Poulter, L. W.: A guide to practical histochemistry. Edinburgh: Oliver & Boyd 1969.
Chayen, J., Bitensky, L., Wells, P. J.: Mitochondrial enzyme latency and its significance in histochemistry and biochemistry. J. roy. micr. Soc. 86, 69–74 (1966).
Farber, E., Bueding, E.: Histochemical localisation of specific oxidative enzymes. V. The dissociation of succinic dehydrogenase from carriers of lipase and the specific histochemical localisation of the dehydrogenase with phenazine methosulphate and tetrazolium salts. J. Histochem. Cytochem. 4, 357–362 (1956).
Farber, E., Louviere, C. D.: Histochemical localisation of specific oxidative enzymes. IV. Soluble oxidation-reduction dyes as aids in the histochemical localization of oxidative enzymes with tetrazolium salts. J. Histochem. Cytochem. 4, 347–356 (1956).
Lehninger, A. L.: The mitochondrion: Molecular basis of structure and function, p. 70. New York: Benjamin 1965.
Silcox, A. A., Poulter, L. W., Bitensky, L., Chayen, J.: An examination of some factors affecting histological preservation in frozen sections of unfixed tissue. J. roy. micr. Soc. 84, 559–564 (1965).
Singer, T. P., Kearney, E. B.: Solubilisation, assay and purification of succinic dehydrogenase. Biochim. biophys. Acta (Amst.) 15, 151–153 (1954).
Slater, T. F.: Studies on a succinate-neotetrazolium reductase system of rat liver. II. Points of coupling with the respiratory chain. Biochim. biophys. Acta (Amst.) 77, 365–382 (1963).
Thorn, M. B.: Inhibition by malonate of succinic dehydrogenase in heart-muscle preparations. Biochem. J. 54, 540–547 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butcher, R.G. Succinate oxidation in intact tissue sections: the effect of oxidation in the cytoplasm. Histochemie 32, 369–378 (1972). https://doi.org/10.1007/BF00305771
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00305771