Summary
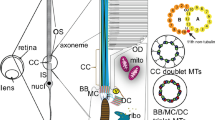
The connecting cilium of the rabbit photoreceptor rod is composed of nine outer doublets, lacking dynein side arms. The central singlet microtubules are absent. In cross section, there is an inner dense ring situated between the doublets and the center core of the cilium. As the doublet microtubules progress from the connecting region into outer segments, the cylindrical array of the nine pairs of doublets spreads out as a brush-like arrangement into the incisure cavity of the outer segment. The microtubules continue as doublets for much of the length of the outer segment. The B-tubules terminate first; the A-tubules extend as single tubules into the apical region of the photoreceptor. Before the B-tubules end, they open up, forming hook-shaped projections from the A-tubules. The gradual reduction in length of these hook-shaped structures suggests that near their distal ends each B-tubule opens because of the separation of protofilament 1 of the B-tubule from protofilament 1 of the adjacent A-tubule. Subsequently, the B-tubule protofilaments terminate individually.
Similar content being viewed by others
References
Afzelius BA (1979) The immotile-cilia syndrome and other disease. Int Rev Expt Pathol 19:1–43
Amos LA, Klug A (1974) Arrangement of subunits in flagellar microtubules. J Cell Sci 14:523–549
Cohen AI (1965) New details of the ultrastructure of the outer segments and ciliary connections of the rods of human and macaque retinas. Anat Rec 152:63–80
Cote RH, Bergen LG, Borisy GG (1980) Head-to-tail polymerization of microtubules in vitro: A review In: DeBrabander M, De Mey J (eds) Microtubules and microtubule inhibitors, 1980, Elsevier/North-Holland, Amsterdam, pp 325–338
De Robertis E (1956) Electron microscope observations on the submicroscopic organization of the retinal rods. J Biophys Biochem Cytol 2:319–337
De Robertis E (1960) Some observations on the ultrastructure and morphogenesis of photoreceptors. J General Physiol 43:1–13
Dowling JW (1967) The organization of vertebrate visual receptors. In: Allen JM (ed) Molecular organization and biological function, Harper and Rew, New York, pp 186–210
Eakin RM (1961) Photorecetpors in the amphibian frontal organ. Proc Natl Acad Sci (USA) 47:1084–1088
Gilula NB, Satir P (1972) The ciliary necklace. A ciliary membrane specialization. J Cell Biol 53:494–509
Heidemann SR (1980) Visualization of microtubule polarity. In: DeBrabander M, De Mey J (eds) Microtubules and microtubule inhibitors 1980, Elsevier/North-Holland, Amsterdam, pp 341–355
Heidemann SR, McIntosh JR (1980) Visualization of the structural polarity of microtubules. Nature (London) 286:517–519
Margolis RL, Wilson L (1978) Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell 13:1–8
Margolis RL, Wilson L (1981) Microtubule treadmills—possible molecular machinery. Nature (London) 293:705–711
Matsusaka T (1975) A microtubule system of connecting cilium. Acta Soc Ophthalmol Jpn 79:132–137
Matsusaka T (1976) Cytoplasmic fibrils of the connecting cilium. J Ultrastruct Res 54:318–324
McIntosh JR, Euteneuer U, Neighbors B (1980) Intrinsic polarity as a factor in microtubule function. In: DeBrabander M, De Mey J (eds) Microtubules and microtubule inhibitors 1980. Elsevier/North-Holland, Amsterdam, pp 357–371
Nilsson SEG (1964) Receptor cell outer segment development and ultrastructure of the disk membranes in the retina of the tadpole (Rana pipiens). J Ultrastruct Res 11:581–602
Reynolds ES (1963) Electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Ringo DL (1967) Flagellar motion and fine structure of the flagellar aparatus in Chlamydomonas. J Cell Biol 33:543–571
Rosenbaum JL, Child FM (1967) Flagellar regeneration in protozoan flagellates. J Cell Biol 34:345–364
Rosenbaum JL, Moulder JE, Ringo DL (1969) Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol 41:600–619
Rosenbaum JL, Binder, LI, Granett S, Dentler WL, Snell W, Sloboda R, Haimo L (1975) Directionality and rate of assembly of chick brain tubulin onto pieces of neurotubules, flagellar axonemes and basal bodies. Ann NY Acad Sci 253:147–177
Sjöstrand FS (1953a) The ultrastructure of the outer segments of rods and cones of the eye as revealed by the electron microscope. J Cell Comp Physiol 42:15–44
Sjöstrand FS (1953b) The ultrastructure of the inner segments of the retinal rods of the guinea pig eye as revealed by electron microscopy. J Cell Comp Physiol 42:45–70
Soifer D (1982) Assembly and disposition of microtubule proteins: some boundary conditions for possible role of microtubules in axoplasmic transport. In: Weiss D (ed) Axoplasmic transport. Springer, Berlin Heidelberg New York, pp 81–90
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Summers K, Kirschner MW (1979) Characteristics of the polar assembly and disassembly of microtubules observed in vitro by darkfield light microscopy. J Cell Biol 83:205–217
Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Synder DH (1973) Microtubules: Evidence for 13 protofilaments. J Cell Biol 59:267–275
Warner FD, Satir P (1974) The structural basis of ciliary bend formation: Radial spoke positional changes accompanying microtubule sliding. J Cell Biol 63:35–63
Wisniewski HM, Bloom BR (1975) Experimental allergic optic neuritis (EAON) in the rabbit: A new model to study primary demyelinating diseases. J Neurol Sci 24:257–263
Witman GB (1975) The site of in vivo assembly of flagellar microtubules. Ann NY Acad Sci 253:178–191
Witman GB, Carlson K, Berliner J, Rosenbaum JL (1972a) Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes and mastigonemes. J Cell Biol 54:507–539
Witman GB, Carlson K, Rosenbaum JL (1972b) Chlamydomonas flagella. II. The distribution of tubulins 1 and 2 in the outer doublet microtubules. J Cell Biol 54:540–550
Yacob A, Wise C, Kuntz YW (1977) The accessory outer segment of rods and cones in the retina of guppy, Poecilia reticulata (Teleostei): An electron microscopical study. Cell Tissue Res 177:181–193
Young RW (1968) Passage of newly formed protein through the connecting cilium of retinal rods in the frog. J Ultrastruct Res 23:462–473
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wen, G.Y., Soifer, D. & Wisniewski, H.M. The doublet microtubules of rods of the rabbit retina. Anat Embryol 165, 315–328 (1982). https://doi.org/10.1007/BF00305570
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305570