Summary
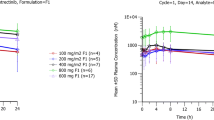
The effect of dose on the bioavailability of oral etoposide was investigated in ten patients with malignant mesothelioma who received single-agent etoposide as part of a phase II study. Etoposide pharmacokinetics were studied in each patient at oral dose levels of 100, 200, 300, 400 and 600 mg. At doses above 200 mg, the AUC and peak concentrations of etoposide were substantially lower than predictions based on the 100-mg dose. This study confirms previous observations that etoposide absorption is dose-dependent and that a mean bioavailability of approximately 50% cannot be assumed at total oral doses >200 mg.
Similar content being viewed by others
References
Allen LM, Tejada F, Okonmah A, Nordqvist S (1982) Combination chemotherapy of the epipodophyllotoxin derivates teniposide and etoposide. Cancer Chemother Pharmacol 7: 151–156
Arnold AM (1979) Podophyllotoxin derivative VP16-213. Cancer Chemother Pharmacol 3: 71
Arnold AM, Whitehouse JMA (1981) Etoposide: a new anticancer agent. Lancet ii: 912
Cavalli F, Sontagg RW, Jungi F, Senn HJ, Brunner KW (1976) VP16 monotherapy for remission induction of small cell lung cancer. A randomised trial using three dosage schedules. Cancer Treat Rep 62: 463–475
Cavalli F (1982) VP16-213 (etoposide). A critical review of its activity. Cancer Chemother Pharmacol 7: 81
D'Incalci M, Farina P, Sessa C, Mangioni C, Conter V, Masera G, Rocchetti M, Pisoni MB, Piazza E, Beer M, Cavalli F (1982) Pharmacokinetics of VP16-213 given by different administration methods. Cancer Chemother Pharmacol 7: 141
Hande KR, McKay CM, Wedlund PJ, Noone RM, Shea WK, Fer MF, Greco FA, Wolff SN (1982) Clinical pharmacology of high dose VP16-213. (Abstract 513) Proc Am Assoc Cancer Res 23: 131
Harvey VJ, Slevin ML, Joel SP, Ang LM, Johnston A, Barnett MJ, Wrigley PFM (1984) The pharmacokinetics of VP16 (etoposide) and bioavailability following different methods of administration. (Abstract) Br J Clin Pharmacol 17: 204
Harvey VJ, Joel SP, Johnston A, Slevin ML (1985) High-performance liquid chromatography of etoposide in plasma and urine. J Chromatogr 339: 419
Harvey VJ, Slevin ML, Joel SP, Johnston A, Wrigley PFM (1986) The effect of dose on the bioavailability of oral etoposide. Cancer Chemother Pharmacol 16: 178–181
Holthuis JJM, Postmus PE, Sleiifer DT, Mulder NH, Verleun H, van Oort WJ (1983) Pharmacokinetics of etoposide (VP16-213) after high dose intravenous administration. (Abstract) Proceedings of the Second European Conference on Clinical Oncology and Cancer Nursing, Amsterdam, p 13
Issell BF, Crooke ST (1979) Etoposide (VP16-213). Cancer Treat Rev 6: 107
Johnston A, Woollard RC (1983) STRIPE: an interactive computer program for the analysis of drug pharmacokinetics. J Pharmacol Methods 9: 193
Karnofsky DA, Abelman WH, Craver LF, Buchenal JH (1948) The use of the nitrogen mustards in the palliative treatment of carcinoma with particular reference to broncheogenic carcinoma. Cancer 1: 634
Nissen NI, Dombernowsky P, Hansen HH, Pederson AG (1980) The epipodophyllotoxin derivatives VM-26 and VP16-213, 1976–1979, a review. Recent Results Cancer Res 74: 98
Rozencweig M, von Hoff DD, Henney JE, Muggia FM (1977) VM26 and VP16-213, a comparative analysis. Cancer 40: 334
Slevin ML, Harvey VJ, Joel SP, Smythe MM, Johnston A, Wrigley PFM (1983) Variable absorption following repeated oral doses of VP-16 an epipodophyllotoxin. (Abstract) Proceedings of the Second European Conference on Clinical Oncology and Cancer Nursing, Amsterdam, p 11
Slevin ML, Clark PI, Osborne RJ, Malik S, Wood CD, Harvey VJ, Joel SP, Malpas JS, Wrigley PFM (1986) A randomised trial to evaluate the effect of schedule on the activity of etoposide in small cell lung cancer. (Abstract) Proc Am Assoc Clin Oncol 5: 685
Stahelin H (1973) Activity of a new glycosidic lignan derivative (VP16-213) related to podophyllotoxin in experimental tumours. Eur J Cancer 9: 215–221
Vogelzwang NJ, Raghavan D, Kennedy BJ (1982) VP16-213 (etoposide). The mandrake root from Issyk-Kul. Am J Med 72–136
Wolff SN, Fer MF, McKay CM, Hande KR, Hainsworth JD, Greco AF (1983) High-dose VP16-213 and autologous bone marrow transplantation for refractory malignancies: phase I study. J Clin Oncol 1: 701
Wolff SN, Johnson DH, Hande KR, Hainsworth JD, Greco JD (1983) High dose etoposide as single-agent chemotherapy for small cell carcinoma of the lung. Cancer Treat Rep 67: 957
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Slevin, M.L., Joel, S.P., Whomsley, R. et al. The effect of dose on the bioavailability of oral etoposide: confirmation of a clinically relevant observation. Cancer Chemother. Pharmacol. 24, 329–331 (1989). https://doi.org/10.1007/BF00304768
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304768