Summary
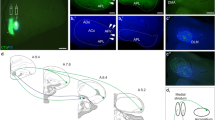
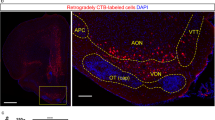
The anterior dorsal ventricular ridge (ADVR), a large intraventricular protrusion in the reptilian forebrain, receives information from many different sensory modalities and in turn, projects massively onto the striatum. The ADVR possesses functional similarities to the mammalian isocortex and may perform complex sensory integrations. The ADVR in lizards is composed of three longitudinal zones which receive visual, somatosensory and acustic information, respectively. These projections are relayed via thalamic nuclei. Previous retrograde tracer studies also suggested brain stem projections to the ADVR arising in the midbrain reticular formation and in certain monoaminergic brain stem nuclei (substantia nigra, locus coeruleus and nucleus raphes superior). In the present study the powerful retrograde fluorescent tracer. ‘Fast Blue’ was applied as a slow-release gel to the ADVR of the savanna monitor lizard, Varanus exanthematicus. Thalamic projections were confirmed and various direct brain stem projections to the ADVR were demonstrated. Brain stem afferents to the ADVR were found from the laminar nucleus of the torus semicircularis (possibly comparable to the mammalian periaqueductal gray), from the midbrain reticular formation, from the substantia nigra (pars compacta and reticulata) and the adjacent ventral tegmental area, from the nucleus raphes superior, from the locus coeruleus, from the parabrachial region, from the nucleus of the lateral lemniscus and even from the most caudal part of the brain stem (a few neurons in the nucleus of the solitary tract and lateral reticular formation, possibly comparable to the mammalian A2 and A1 groups, respectively). These data strongly suggest direct ADVR projections from the parabrachial region (related to visceral and taste information) as well as distinct catecholaminergic (presumably dopaminergic: substantia nigra, ventral tegmental area and, noradrenergic: locus coeruleus, respectively) and serotonergic projections (nucleus raphes superior).
Similar content being viewed by others
References
Balaban CD, Ulinski PS (1981) Organization of thalamic afferents to anterior dorsal ventricular ridge in turtles. I. Projections of thalamic nuclei. J Comp Neurol 200:95–129
Belekhova MG, Kenigfest NB (1983) A study of the hippocampal mediodorsal cortex connections in lizards by means of horseradish peroxidase axonal transport. Neurophysiology (Kiev) 15:145–152
Belekhova MG, Zharskaja VD, Khachunts AS, Gaidaenko GV, Tumanova NL (1985) Connections of the mesencephalic, thalamic and telencephalic auditory centers in turtles. Some structural bases for audiosomatic interrelations. J Hirnforsch 26:127–152
Bentivoglio M, Macchi G, Rossini P, Tempesta E (1978) Brain stem neurons projecting to neocortex: a HRP study in the cat. Exp Brain Res 31:489–498
Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T (eds) Handbook of Chemical Neuroanatomy, vol 2: Classical Transmitters in the CNS. Elsevier, Amsterdam, pp 55–122
Bruce LL, Butler AB (1984a) Telencephalic connections in lizards. I. Projections to cortex. J Comp Neurol 229:585–601
Bruce LL, Butler AB (1984b) Telencephalic connections in lizards. II. Projections to anterior dorsal ventricular ridge. J Comp Neurol 229:602–615
Carpenter MB (1981) Anatomy of the corpus striatum and brain stem integrating systems. In: Brooks VB (ed) Handbook of Physiology-The Nervous System, Vol II (Motor Control). Am Physiol Soc, Bethesda, pp 947–995
Desan PH (1985) Afferent connections of the cerebral cortex of the turtle (Pseudemys scripta elegans). Soc Neurosci Abst 11:1310
Divac I (1975) Magnocellular nuclei of the basal forebrain project to neocortex, brain stem, and olfactory bulb. Review of some functional correlates. Brain Res 93:385–398
Fulwiler CE, Saper CB (1984) Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Rev Rev 7:229–259
Graybiel AM, Ragsdale CW (1983) Biochemical anatomy of the striatum. In: Emson PC (ed) Chemical Neuroanatomy. Raven Press, New York, pp 427–504
Griffin G, Watkins LR, Mayer DJ (1979) HRP pellets and slowrelease gels: two new techniques for greater localization and sensitivity. Brain Res 168:595–601
Hohmann CF, Carroll PT, Ebner FF (1983) Acetylcholine levels and choline acetyltransferase activity in turtle cortex. Brain Res 258:120–122
Hoogland PV (1977) Efferent connections of the striatum in Tupinambis nigropunctatus. J Morphol 152:229–246
Hoogland PV (1981) Spinothalamic projections in a lizard, Varanus exanthematicus: an HRP study. J Comp Neurol 198:7–12
Jones BE, Yang T-Z (1985) The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242:56–92
Källén B (1951) On the ontogeny of the reptilian forebrain. Nuclear structures and ventricular sulci. J Comp Neurol 36:143–192
Kievit J, Kuypers HGJM (1975) Subcortical afferents to the frontal lobe in the rhesus monkey studied by means of retrograde horseradish peroxidase transport. Brain Res 85:261–266
Kimura H, McGeer PL, Peng JH, McGeer EG (1981) The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol 200:151–201
Kuypers HGJM, Huisman AM (1984) Fluorescent neuronal tracers. In: Federoff S (ed) Labeling Methods Applicable to the Study of Neuronal Pathways. Adv Cell Neurobiol, vol 5. Academic Press, New York, pp 307–340
Lasiter PS, Glanzman DL, Mensah PA (1982) Direct connectivity between pontine taste areas and gustatory neocortex in rat. Brain Res 234:111–121
Lindvall O, Björklund A (1978) Organization of catecholamine neurons in the rat central nervous system. In: Iversen LL, Iversen SD, Snyder SH (eds) Chemical Pathways in the Brain. Hb Psychopharmacol, vol 9. Plenum Press, New York, pp 139–231
Lohman AHM, van Woerden-Verkley I (1978) Ascending connections to the forebrain in the tegu lizard. J Comp Neurol 182:555–594
Loughlin SE, Fallon JH (1984) Substantia nigra and ventral tegmental area projections to cortex: topography and collateralization. Neuroscience 11:425–435
Markowitsch HJ, Irle E (1981) Widespread cortical projections of the ventral tegmental area and of other brain stem structures in the cat. Exp Brain Res 41:233–246
Mesulam M-M, Mufson EJ, Levey AI, Wainer BH (1983) Cholinergic innervation of cortex by basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata) and hypothalamus in the rhesus monkey. J Comp Neurol 214:170–197
Mufson EJ, Desan PH, Mesulam M-M, Wainer BH, Levey AI (1984) Choline aceyltransferase-like immunoreactivity in the forebrain of the red-eared pond turtle (Pseudemys scripta elegans). Brain Res 323:103–108
Nauta WJH, Karten HJ (1970) A general profile of the vertebrate brain, with sidelights on the ancestry of cerebral cortex. In: Schmitt FO (ed) The Neurosciences: Second Study Program. Rockefeller University Press, New York, pp 7–26
Nieuwenhuys R (1985) Chemoarchitecture of the Brain. Springer Verlag, Berlin Heidelberg New York
Norgren R (1984) Taste: central neural mechanisms. In: Darian-Smith I (ed) Hb Physiology. The Nervous System, Vol III (Sensory Processes). Am Physiol Soc, Bethesda, pp 1087–1128
Northcutt RG (1978) Forebrain and midbrain organization in lizards and its phylogenetic significance. In: Greenberg N, MacLean PD (eds) Behavior and Neurology of Lizards, Nat Inst Mental Health, Rockville, Md, pp 11–64
Northeutt RG (1981) Evolution of the telencephalon in nonmammals. Ann Rev Neurosci 4:301–350
Northcutt RG (1984) Evolution of the vertebrate central nervous system: patterns and processes. Am Zool 24:701–716
Oades RD, Halliday GM (1987) Ventral tegmental (A10)system: neurobiology. 1. Anatomy and connectivity. Brain Res Rev 12:117–165
Ouimet CC, Patrick RL, Ebner FF (1985) The projection of three extrathalamic cell groups to the cerebral cortex of the turtle Pseudemys. J Comp Neurol 237:77–84
Parent A (1979) Monoaminergic systems of the brain. In: Gans C, Northcutt RG, Ulinski P (eds) Biology of the Reptilia, vol 10: Neurology B. Academic Press, London, pp 247–285
Parent A, Poitras D, Dubé L (1984) Comparative anatomy of central monoaminergic systems. In: Björklund A, Hökfelt T (eds) Handbook of Chemical Neuroanatomy, vol 2: Classical Transmitters in the CNS. Elsevier, Amsterdam, pp 409–439
Porrino LJ, Goldman-Rakic PS (1982) Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol 205:63–76
Pritz MB (1980) Parallels in the organization of auditory and visual systems in crocodiles. In: Ebbesson SOE (ed) Comparative Neurology of the telencephalon. Plenum Press, New York, pp 331–342
Pritz MB, Northcutt RG (1980) Anatomical evidence for an ascending somatosensory pathway to the telencephalon in crocodile, Caiman corocilus. Exp Brain Res 40:342–345
Pritz MB, Stritzel ME (1985) Thalamic projections to the noncortical telencephalon in a reptile. Soc Neurosci Abstr 11:1309
Ricardo JA, Koh ET (1978) Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153:1–26
Smeets WJAJ, Hoogland PV, Voorn P (1986) The distribution of dopamine immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko: an immunohistochemical study with antibodies against dopamine. J Comp Neurol 253:46–60
Steinbusch HWM, Nieuwenhuys R (1983) The raphe nuclci of the rat brain stem: a cytoarchitectonic and immunohistochemical study. In: Emson PC (ed) Chemical Neuroanatomy. Raven Press, New York, pp 131–207
ten Donkelaar HJ, de Boer-van Huizen R (1981) Ascending projections of the brain stem reticular formation in a nonmammalian vertebrate (the lizard Varanus exanthematicus), with notes on the afferent connections of the forebrain. J Comp Neurol 200:501–528
ten Donkelaar HJ, de Boer-van Huizen R (1984) Ascending and descending axon collaterals efferent from the brain stem reticular formation. A retrograde fluorescent tracer study in the lizard, Varanus exanthematicus. Brain Res 32:184–188
ten Donkelaar HJ, Bangma GC, de Boer-van Huizen R (1985) The fasciculus longitudinalis medialis in the lizard Varanus exanthematicus. 2. Vestibular and internuclear components. Anat Embryol 172:205–215
ten Donkelaar HJ, Bangma GC, Barbas-Henry HA, de Boer-van Huizen R, Wolters JG (1987) The brain stem in a lizard Varanus exanthematicus. Adv Anat Embryol Cell Biol, Vol 107. Springer, Berlin Heidelberg New York
Ueda S, Takeushi Y, Sano Y (1983) Immunohistochemical demonstration of serotonin neurons in the central nervous system of the turtle Clemmys japonica. Anat Embryol 108:1–19
Ulinski PS (1983) Dorsal Ventricular Ridge: A Treatise on Forebrain Organization in Reptiles and Birds. Wiley, New York
Voneida TJ, Sligar CM (1979) Efferent projections of the dorsal ventricular ridge and the striatum in the tegu lizard, Tupinambis nigropunctatus. J Comp Neurol 186:43–64
Wolters JG, ten Donkelaar HJ, Verhofstad AAJ (1984) Distribution of catecholamines in the brain stem and spinal cord of the lizard Varanus exanthematicus: an immunohistochemical study based on the use of antibodies to tyrosine hydroxylase. Neuroscience 13:469–493
Wolters JG, ten Donkelaar HJ, Steinbusch HWM, Verhofstad AAJ (1985) Distribution of serotonin in the brain stem and spinal cord of the lizard Varanus exanthematcus: an immunohistochemical study. Neuroscience 14:169–193
Wolters JG, ten Donkelaar HJ, Verhofstad AAJ (1986a) Distribution of some peptides (substance P, Leu enkephalin, Met enkephalin) in the brain stem and spinal cord of a lizard, Varanus exanthematicus. Neuroscience 18:917–946
Wolters JG, de Boer-van Huizen R, ten Donkelaar HJ, Leenen L (1986b) Collateralization of descending pathways from the brainstem to the spinal cord in a lizard, Varanus exanthematicus. J Comp Neurol 251:317–333
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ten Donkelaar, H.J., de Boer-van Huizen, R. Brain stem afferents to the anterior dorsal ventricular ridge in a lizard (Varanus exanthematicus). Anat Embryol 177, 465–475 (1988). https://doi.org/10.1007/BF00304745
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304745