Summary
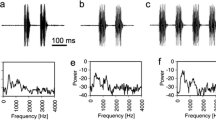
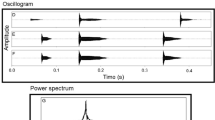
The vocal behavior of Hyla versicolor was studied in the field by means of behavioral observations and playback experiments, and these data were coupled with measurements of oxygen consumption in calling frogs to estimate the effect of social interactions on calling energetics. Male gray treefrogs have intense calls (median peak SPL=109 dB, fast RMS SPL=100 dB at 50 cm). At an air temperature of 23° C, males produced an average of 1,200–1,300 calls/h for 2–4 h per night. Calling rates and call durations differed among individuals, but were relatively constant for each male during periods of sustained calling. Males in dense choruses gave calls about twice as long as isolated males, but produced calls at about half the rate. Consequently, total calling effort and estimated aerobic costs were largely independent of chorus density. Playbacks of recorded calls to males in the field elicited increases in call duration and decreases in calling rate, regardless of the rate or duration of the stimulus. Males gave longer calls in response to long calls or to stimuli presented at high rates, but they did not precisely match either stimulus rate or duration. Calling effort and estimated oxygen consumption changed only slightly during stimulus playbacks. These results indicate that male-male competition elicits pro-found changes in the vocal behavior of calling males, but these changes have little effect on energy expenditure. We estimated that most calling males had metabolic rates of about 1.7–1.8 ml O2/(g\h), or about 280 J/h for an average size (8.6 g) male at 20° C. Although changes in call duration and calling rate did not affect aerobic costs of calling, males producing long calls at slow rates called for fewer hours per night than males producing shorter calls at higher rates. This suggests that calling time may be limited by the rate at which muscle glycogen reserves are depleted.
Similar content being viewed by others
References
Alexander RD (1975) Natural selection and specialized chorusing behavior in acoustical insects. In: Pimentel D (ed) Insects, science and society. Academic Press, New York, pp 35–77
Andersson M (1982) Sexual selection, natural selection and quality advertisement. Biol J Linn Soc 17:375–393
Arak A (1983a) Male-male competition and mate choice in anuran amphibians. In: Bateson P (ed) Mate choice. Cambridge University Press, New York, pp 181–210
Arak A (1983b) Sexual selection by male-male competition in natterjack toad choruses. Nature 306:261–262
Bergstrom JW, Hermansen L, Hultman E, Saltin B (1967) Diet, muscle glycogen and physical performance. Acta Physiol Scand 71:140–150
Bradbury JW, Gibson R (1983) Leks and mate choice. In: Bateson P (ed) Mate choice. Cambridge University Press, New York, pp 109–138
Bucher TL, Ryan MJ, Bartholomew GA (1982) Oxygen consumption during resting, calling, and nest building in the frog Physalaemus pustulosus. Physiol Zool 55:10–22
Essén B (1978) Glycogen depletion of different fibre types in human skeletal muscle during intermittent and continuous exercise. Acta Physiol Scand 103:446–455
Farr JA (1980) Social behavior patterns as determinants of reproductive success in the guppy, Poecilia reticulata Peters (Pisces: Poeciliidae). Behaviour 74:38–91
Fellers GM (1979) Aggression, territoriality, and mating behavior in North American treefrogs. Anim Behav 27:107–119
Forester DC, Czarnowsky (1985) Sexual selection in the spring peeper, Hyla crucifer (Amphibia, Anura): role of the advertisement call. Behaviour 92:112–128
Gayou DC (1984) Effects of temperature on the mating call of Hyla versicolor. Copeia 1984:733–738
Gerhardt HC (1975) Sound pressure levels and radiation patterns of the vocalizations of some North American frogs and toads. J Comp Physiol 102:1–12
Gerhardt HC (1978) Temperature coupling in the vocal communication system of the gray tree frog, Hyla versicolor. Science 199:992–994
Greenfield MD, Shaw KC (1983) Adaptive significance of chorusing with special reference to the Orthoptera. In: Gwynne DT, Morris GK (eds) Orthopteran mating systems: sexual competition in a diverse group of insects. Westview Press, Doulder, Colorado, pp 1–27
Heath JE, Josephson RK (1970) Body temperature and singing in the katydid, Neoconocephalus robustus (Orthoptera, Tettigoniidae). Biol Bull 138:272–285
Mac Nally RC (1984) On the reproductive energetics of chorusing males: costs and patterns of call production in two sympatric species of Ranidella (Anura). Oikos 42:82–91
Mac Nally RC, Young D (1981) Song energetics of the bladder cicada, Cystosoma saundersii. J Exp Biol 90:185–196
Marsh RL, Taigen TL (1984) Contractile, biochemical, and structural properties of the muscles used for calling by tree frogs. Am Zool 24:38A
Parker GA (1982) Phenotype limited evolutionarily stable strategies. In: Kings College Sociobiology Group (eds) Current problems in sociobiology. Cambridge University Press, New York, pp 173–201
Parker GA (1983) Mate quality and mating decisions. In: Bateson P (ed) Mate choice. Cambridge University Press, New York, pp 141–164
Prestwich KN, Walker TJ (1981) Energetics of singing in crickets: effect of temperature in three trilling species (Orthoptera: Gryllidae). J Comp Physiol 143:199–212
Rand AS, Ryan MJ (1981) The adaptive significance of a complex vocal repertoire in a neotropical frog. Z Tierpsychol 57:209–214
Ryan MJ (1985) The tungara frog: a study in sexual selection and communication. University of Chicago Press, Chicago
Ryan MJ, Bartholomew GA, Rand AS (1983) Energetics of reproduction in a neotropical frog, Physalaemus pustulosus. Ecology 64:1456–1462
Schwartz JJ, Wells KD (1984) Vocal behavior of the neotropical treefrog Hyla phlebodes. Herpetologica 40:452–463
Schwartz JJ, Wells KD (1985) Intra-and interspecific vocal behavior of the neotropical treefrog Hyla microcephala. Copeia 1985:27–38
Siegel S (1956) Nonparametric statistics for the behavioral sciences. McGraw Hill, New York
Stevens ED, Josephson RK (1977) Metabolic rate and body temperature in singing katydids. Physiol Zool 50:31–42
Sullivan BK (1983) Sexual selection in Woodhouse's toad (Bufo woodhousei). II. Female choice. Anim Behav 31:1011–1017
Taigen TL, Wells KD (1985) Energetics of vocalization by an anuran amphibian (Hyla versicolor). J Comp Physiol B 155:163–170
Taigen TL, Wells KD, Marsh RL (1985) The enzymatic basis of high metabolic rates in calling frogs. Physiol Zool 58:719–726
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Cambridge, Massachusetts
Wells KD, Schwartz JJ (1984) Vocal communcation in a neotropical treefrog, Hyla ebraccata: advertisement calls. Anim Behav 32:405–420
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wells, K.D., Taigen, T.L. The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19, 9–18 (1986). https://doi.org/10.1007/BF00303837
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00303837