Summary
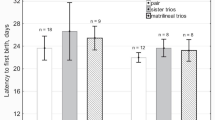
Cnemidophorous uniparens, an all-female, parthenogenetic species of whiptail lizard (Teiidae), were housed in the laboratory under seminatural conditions in groups of four, in pairs, or as isolates. Group structure and an animal's position in the dominance hierarchy affect reproductive effort. Housed in pairs, the dominant animals exhibit a higher rate of reproduction, as measured by number of clutches and length of clutch cycle, than do their subordinate cagemates. Females housed with dihydrotestosterone-treated ovariectomized females lay more clutches than females housed with ovariectomized hormone-untreated females, those housed with intact females, or housed alone.
Similar content being viewed by others
References
Adler NT (1974) The behavioral control of reproductive physiology. Montagna W, Sadler WA (eds) In: Reproductive behavior. Plenum Press, New York, pp 259–286
Brackin MF (1978) The relation of rank to physiological state in Cnemidophorous sexlineatus dominance hierarchies. Herpetologica 34:185–191
Brattstrom BH (1974) The evolution of reptilia social behavior. Am Zool 14:35–50
Cole CJ (1975) Evolution of parthenogenetic species of reptiles. In: Reinboth R (ed) Intersexuality in the animal kingdom, Springer, Berlin Heidelberg New York, pp 340–355
Cole (1978) The value of virgin birth. Nat Hist 87:56–63
Crews D (1974) Effects of group stability, male-male aggression, and male courtship on environmentally induced ovarian recrudescence in the lizard Anolis carolenensis. J Zool 172:419–441
Crews D (1975) Psychobiology of reptilian reproduction. Science 189:1059–1065
Crews D (1980) Interrelationships among ecological, behavioral, and neuroendocrine processes in the reproductive cycle of Anolis carolinensis and other reptiles. In: Roschblatt, JS, Hinde R, Beer G, Busnel MC (eds) Advances in the study of behavior, vol. II. Academic Press, New York, pp 1–76
Crews D, Fitzgerald K (1980) “Sexual” behavior in parthenogenetic lizards (Cnemidophorous). PNAS 77:449–502
Crews D, Gustafson JE, Tokarz RR (1981) Psychobiology of parthenogenesis in reptiles. In: Hvey R, Pianka E, Schoener TW (eds) Lizard ecology symposium. Harvard University Press, Cambridge, MA (in press)
Cuellar O (1971) Reproduction and the mechanism of meiotic restitution in the parthenogenetic lizard Cnemidophorous uniparens. J Morphol 133:139–166
Cuellar O (1974) On the origin of parthenogenesis in vertebrates: The cytological factors. Am Nat 108:625
Cuellar O (1976) Intraclonal histocompatability in a parthenogenetic lizard: Evidence of genetic homogeniety. Science 193:150–153
Cuellar O (1977) Animal parthenogenesis: A new evolutionary-ecological model is needed. Science 197:837–843
Cuellar O (1979) On the ecology of coexistence in parthenogenetic and bisexual lizards of the genus Cnemidophorous. Am Zool 19:773–786
Lehrman DS (1965) Interaction between internal and external environments in the regulation of the reproductive cycle of the ring dove. In: Beach FA (ed) Sex and behavior. John Wiley and Sons, New York, pp 355–380
Leshner AI (1978) An introduction to behavioral endrocrinology. Oxford University Press, Oxford
Lowe CH, Wright JH (1965) The rediscovery of Cnemidophorous arizonae Van Denburgh. J Ariz Acad Sci 3:164–168
Lowe CH, Wright JH (1966) Evolution of parthenogenetic species of Cnemidophorous (whiptail lizards) in western North America. J Ariz Acad Sci 4:81–87
Maslin TP (1962) All-female species of the lizard genus Cnemidophorous Teiidae. Science 135:212–213
Maslin TP (1967) Skin grafting in the bisexual teiid lizard Cnemidophorous tesselatus. J Exp Zool 166:137–149
Maslin TP (1971) Conclusive evidence of parthenogenesis in three species of Cnemidophorous (Teiidae). Copeia 1971:156–158
Serena M (1980) Sex and the single lizard: The origin and evolution of parthenogenetic Cnemidophorous lemniscatus in Surinam. PhD thesis, The University of Colorado
Soumalainen ES (1950) Parthenogenesis in animals. Adv Genet 3:193
Vandenbergh JG (1975) Hormones, pheremones, and behavior. In: Eleftheriou EB, Sprott RL (ed) Hormonal correlates of behavior. Plenum Press, New York, pp 551–584
Wright JH, Lowe CH (1968) Weeds, polyploids, parthenogenesis and the geographical and ecological distribution of all-female species of Cnemidophorous. Copeia 1978:128–138
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gustafson, J.E., Crews, D. Effect of group size and physiological state of a cagemate on reproduction in the parthenogenetic lizard, Cnemidophorous uniparens (Teiidae). Behav Ecol Sociobiol 8, 267–272 (1981). https://doi.org/10.1007/BF00299525
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00299525