Abstract
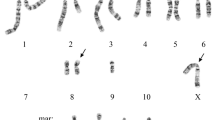
We have investigated the antioxidant properties of V79 Chinese hamster cells rendered resistant to menadione by chronic exposure to increasing concentrations of this quinone. MD1, a clone of resistant cells, was compared to the parental M8 cells; the former showed increased activity of catalase (3 fold), glutathione peroxidase (1.6 fold) and DT-diaphorase (2.6 fold), as well as an increase in glutathione (3.2 fold). Although one of the products of menadione metabolism is superoxide anion, no changes in total superoxide dismutase activity was observed in MD1 cells. MD1 menadione resistant cells were also resistant to killing by hydrogen peroxide and contained tandem duplication of chromosome 6. A similar duplication of chromosome 6 was seen in several independently derived menadione resistant clones and therefore seems closed linked to the establishment of the resistance. Upon removal of menadione from the medium, some of these properties of MD1 cells, viz., resistance to menadione, elevated glutathione levels, and glutathione peroxidase activity, were lost and the cells resembled M8 cells. However, resistance to H2O2, elevated catalase activity and the duplicated chromosome remained stable for more than 40 cell passages in the absence of menadione. The increase in catalase activity was correlated with an increase in catalase mRNA content and a 50% amplification of catalase gene, as determined, respectively, by Northern and Southern blot analysis. The role of the chromosome 6 duplication in resistance to oxidative stress remains to be established. It is not responsible directly for elevated catalase levels since the catalase gene is on chromosome 3.
Similar content being viewed by others
Abbreviations
- SDS:
-
Sodium Dodecyl Sulphate
- SOD:
-
Superoxide Dismutase
- PBS:
-
Phosphate Buffered Saline (8.1 mM Na2HPO4, 1.47 mM KH2PO4, 2.68 mM KCl, 137 mM NaCl)
- CDTA:
-
N,N,N′,N′-tetracetic-trans-1,2-diaminocyclohexane acid
- MOPS:
-
Sulphonic-3-(N-morpholine)-propane acid
- SSC:
-
150 mM Nacl, 15 mM sodium-citrate, pH 6.8
References
Spitz DR, Li GC, McCormick ML, Sun Y, Oberley LW: Stable hydrogen peroxide-resistant variants of CHO fibroblasts demonstrate increases in catalase activity. Radiation Res 114: 114–124, 1988
Krall J, Speranza MJ, Lynch RE: Paraquat-resistant HeLa cells: Increased cellular content of glutathione peroxidase. Arch Biochem Biophys 286: 311–315, 1991
Kelner MJ, Bagnell R: Glutathione-dependent enzymes alone can produce paraquat resistance. Free Radic Biol Med 9: 149–153, 1990
Freedman JH, Ciriolo MR, Peisach J: The role of glutathione in copper metabolism and toxicity. J Biol Chem 264: 5598–5605, 1989
Mello-Filho AC, Chubatsu LS, Meneghini R: V79 Chinese-hamster cells rendered resistant to high cadmium concentration also become resistant to oxidative stress. Biochem J 256: 475–479, 1988
Martins EAL, Meneghini R: DNA damage and lethal effects of hydrogen peroxide and menadione in Chinese hamster cells: distinct mechanisms are involved. Free Radical Biol Med 8: 433–440, 1990
Samuels BL, Murray JL, Cohen MB et al. Increased glutathione peroxidase activity in a human sarcoma cell line with inherent doxorubicin resistance. Cancer Res 51: 521–527, 1991
Kramer RA, Zakher J, Kim G: Role of the glutathione redox cycle in acquired and de novo multidrug resistance. Science 241: 694–697, 1988
Frei B, Winterhalter KH, Richter C: Menadione-(2-methyl-1,4-naphtoquinone)-dependent enzymatic redox cycling and calcium release by mitochondria. Biochemistry 26: 4438–4443, 1986
Ernster, L: DT-diaphorase. In: RW Estabrook, ME Pullmann (eds) Methods in Enzymology, New York and London: Academic Press, 1967, p 309–315
Fox M: Measurement of colony-forming ability and mutagenesis in Chinese hamster cells. In: EC Friedberg, PC Hanawalt (eds) DNA repair, New York: Marcel Dekker, Inc., 1981, p 523–543
Siegel D, Gibson NW, Preusch PC, Ross D: Metabolism of diaziquone by NAD(P)H: (quinone acceptor) oxidoreductase (DT-diaphorase): role of diaziquone-induced DNA damage and cytotoxicity in human colon carcinoma cells. Cancer Res 50: 7293–7300, 1990
Hartree EF: Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48: 422–427, 1972
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ: Protein measurement with the Follin phenol reagent. J Biol Chem 193: 265–275, 1951
Aebi H: Catalase in vitro. Meth Enzymol 105: 114–115, 1984
Oyanagui Y: Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem 142: 290–296, 1984
Flohe L, Gunsler WA: Assays of glutathione peroxidase. Meth Enzymol 105: 114–115, 1984
Akerboom T, Sies H: Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Meth Enzymol 77: 373–382, 1981
Sambrook J, Fritsch EF, Maniatis T: Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press, 1989
Rich PR, Bendall DS: A mechanism for the reduction of cytochromes by quinols in solution and its relevance to biological electron transfer reactions. FEBS Lett 105: 189–194, 1979
Ray M, Mohandas T: Proposed banding nomenclature for the Chinese hamster chromosomes (Crisetus griseus). Cytogenet Cell Genet 16: 83–91, 1976
Stenman G, Sager R: Genetic analysis of tumorogenesis: a conserved region in the human and Chinese hamster genomes contains genetically identified tumor-suppressor genes. Proc Natl Acad Sci USA 84: 9099–9102, 1987
Jones DP, Eklow L, Thor H, Orrenius S: Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated hydrogen peroxide. Arch Biochem Biophys 210: 505–516, 1981
Birnboim HC: Extraction of high molecular weight RNA and DNA from cultured cells. Meth Enzymol 216: 154–160, 1993
Rosen GM, Freeman BA: Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci USA 81: 7269–7273, 1984
Piechaczyc M, Blanchard JM, Marty L, Dani C, Panabieris F, El Sabouti S, Fort P, Jeanteur P: Post-transcriptional regulation of glyceraldehyde-3-phosphatedehydrogenase gene expression in rat tissues. Nucl Acid Res 12: 6951–6963, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martins, E.A.L., Mori, L., Birnboim, H.C. et al. Menadione-resistant Chinese hamster cell variants are cross-resistant to hydrogen peroxide and exhibit stable chromosomal and biochemical alterations. Mol Cell Biochem 116, 181–189 (1992). https://doi.org/10.1007/BF00299397
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00299397