Abstract
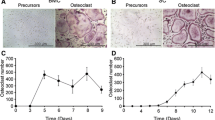
Biochemical and molecular studies of osteoclasts generally require cells in a reasonable degree of purity. The chicken has been extremely useful in this regard, as abundant avian osteoclasts can be generated in vitro entirely from pure populations of marrow macrophage precursors. Propagation of murine osteoclasts is, in contrast, far less efficients, demanding the presence of stromal cells. The aims of this study were to develop a method by which murine osteoclasts generated in culture, can be effectively enriched while maintaining viability and, to explore the mechanisms by which stromal cells promote murine osteoclast generation and survival. We find that 106 fractionated murine marrow cells enriched, for marrow-residing colony-forming units (CFU-cs), yield 3000–4000 tartrate-resistant acid phosphatase (TRAP)-expressing multinucleated giant cells when cultured for 12 days with ST-2 stromal cells. These cells are osteoclasts as evidenced by their ability to “pit” bone slices, resorb radiolabeled bone particles, and generate cyclic AMP in response to calcitonin. Treatment of these generated osteoclast cultures with bacterial collagenase for 2 hours at 37° selectively removes virtually all ST-2 cells, yielding a >60% pure population of TRAP and calcitonin receptor-expressing cells, 90% of which are viable. These cells continue to respond to calcitonin and survive for 24 hours in the absence of ST-2 cells. We also found that murine osteoclast generation depends upon contact of osteoclast precursors with viable ST-2 cells. Furthermore, the stromal cells secrete macrophage colony-stimulating factor (CSF-1), and the anti-CSF-1 antibody 5A1 inhibits murine osteoclastogenesis. Exogenous CSF-1, in turn, partially overrides the anti-osteoclastogenic effect of 5A1. We conclude that (1) the purity of murine osteoclasts generated from bone marrow cells enriched for CFU-cs can be greatly enhanced by selective removal of associated stromal cells, and (2) both soluble and membraneresiding stromal cell factors are necessary for generation of murine osteoclasts.
Similar content being viewed by others
References
Coccia PF, Krivit W, Cervenka J, Clawson CC, Kersey JH, Kim TH, Nesbit ME, Ramsay NKC, Warkentin PI, Teitelbaum SL, Kahn AJ, Brown DM (1980) Successful bone marrow transplantation for infantile malignant osteopetrosis. N Engl J Med 302:701–708
Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchu A, Kodama H, Martin TJ, Suda T (1989) The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclastlike cell differentiation in cocultures with mouse spleen cells. Endocrinology 125:1805–1813
Udagawa N, Takahashi N, Akatsu H, Tanaka T, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T (1990) Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA 87:7260–7264
Alvarez JI, Teitelbaum SL, Blair HC, Greenfield EM, Athanasou NA, Ross FP (1991) Generation of avian cells resembling osteoclasts from mononuclear phagocytes. Endocrinology 128:2324–2335
Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH (1983) CSF-1—a mononuclear phagocyte lineage-specific hematopoietic growth factor. J Cell Biochem 21:151–159
Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Ahmed-Ansara A, Sell KW, Pollard JW, Stanley ER (1990) Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA 87:4828–4832
Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H (1990) Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res 5:781–89
Felix R, Cecchini MG, Fleish H (1990) Macrophage colony-stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology 127:2592–2594
Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, Kurokawa T, Suda T (1993) Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest 91:257–263
Lokeshwar BL, Lin H-S (1988) Development and characterization of monoclonal antibodies to murine macrophage colony-stimulating factor. J Immunol 141:483–488
Stanley ER, Guilbert LJ (1981) Methods for the purification, assay, characterization and target cell binding of a colony-stimulating factor (CSF-1). J Immunol Methods 42:253–284
Clohisy DR, Bar-Shavit Z, Chappel J, Teitelbaum SL (1987) 1,25-dihydroxyvitamin D3 modulates bone marrow macrophage precursor proliferation and differentiation: upregulation of the mannose receptor. J Biol Chem 262:15922–15929
Perkins SL, Teitelbaum SL (1991) 1,25-diydroxyvitamin D3 modulates colony-stimulating factor-1 receptor binding by murine bone marrow macrophage precursors. Endocrinology 128:303–311
Arnett TR, Dempster DW (1987) A comparative study of disaggregated chick and rat osteoclasts in vitro: effects of calcitonin and prostaglandins. Endocrinology 120:602–608
Blair HC, Kahn AJ, Crouch EC, Jeffrey JJ, Teitelbaum SL (1986) Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol 102:1164–1172
Zambonin-Zallone A, Teti A, Primavera MV (1982) Isolated osteoclasts in primary culture: first observations on structure and survival in culture media. Anat Embryol 165:405–413
Nicholson GC, Moseley JM, Sexton PM, Martin TJ (1987) Chicken osteoclasts do not possess calcitonin receptors. J Bone Miner Res 2:53–59
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shioi, A., Ross, F.P. & Teitelbaum, S.L. Enrichment of generated murine osteoclasts. Calcif Tissue Int 55, 387–394 (1994). https://doi.org/10.1007/BF00299320
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00299320