Abstract
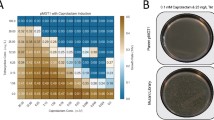
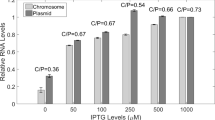
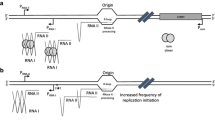
A new chromosomal mutation (cpeA), that causes increased expression of plasmid-borne genes in Escherichia coli, was identified and mapped to the sbcB locus. The effect of the mutation on plasmid transcription was non-specific with respect to the various promoters that were studied, but was more pronounced for an lncW low-copy-number plasmid than for ColEl- or pl5A-based, high-copy-number plasmids. The mutant phenotype was observed even in recB + C + D + strains, but not in recA mutants. The increased-expression phenotype was also observed in sbcB15 but not in xonA1 (another sbcB allele) mutants, suggesting that the expression of this phenotype is mediated by genes of the so-called RecF pathway family. Consistent with this interpretation was the observation that the cpeA mutant phenotype was less pronounced in recF, recJ and recO mutants. The increased-expression phenotype was also correlated with increased recovery of plasmid DNA from the cpeA/sbcB mutant strains, but there was no evidence for the occurrence of linear plasmid multimers in these strains.
Similar content being viewed by others
References
Allgood ND, Silhavy TJ (1991) Escherichia coli xonA (sbcB) mutants enhance illegitimate recombination. Genetics 127:671–680
Andrews AE, Lawley B, Pittard AJ (1991) Mutational analysis of repression and activation of the tyrP gene in Escherichia coli. J Bacteriol 173:5068–5078
Auble DT, Allen TL, deHaseth PL (1986) Promoter recognition by Escherichia coli RNA polymerase. J Biol Chem 261:11202–11206
Bachmann BJ (1990) Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev 54:130–197
Barbour SD, Nagaishi H, Templin A, Clark AJ (1970) Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of Rec mutations. Proc Natl Acad Sci USA 67:128–135
Biek DP, Cohen SN (1992) Propagation of pSC101 plasmids defective in binding of integration host factor. J Bacteriol 174:785–792
Capaldo-Kimball F, Barbour SD (1971) Involvement of recombination genes in growth and viability of E. coli K-12. J Bacteriol 106:204–212
Clark AJ (1991) rec genes and homologous recombination proteins in Escherichia coli. Biochimie 73:523–532
Clark AJ, Low KB (1988) Pathways and systems of homologous recombination in Escherichia coli. In: Low K (ed) The recombination of genetic material. Academic Press, San Diego, pp 155–215
Clark AJ, Margulies AD (1965) Isolation and characterization of recombination-deficient mutants of Escherichia coli K-12. Proc Natl Acad Sci USA 53:451–459
Clark AJ, Sandler SJ, Willis DK, Chu CC, Blanar MA, Lovett ST (1984) Genes of the RecE and RecF pathways of conjugational recombination in Escherichia coli. Cold Spring Harbor Symp Quant Biol 49:453–462
Cohen A, Clark AJ (1986) Synthesis of linear plasmid multimers in Escherichia coli K-12. J Bacteriol 167:327–335
Dattananda CS, Rajkumari K, Gowrishankar J (1991) Multiple mechanisms contribute to osmotic inducibility of pro U operon expression in Escherichia coli: demonstration of two osmoresponsive promoters and of a negative regulatory element within the first structural gene. J Bacteriol 173:7481–7490
Dunn JJ, Studier FW (1983) Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol 166:477–535
Emmerson PT, Howard-Flanders P (1967) Cotransduction with thy of a gene required for genetic recombination in Escherichia coli. J Bacteriol 93:1729–1731
Gillen JR, Willis DK, Clark AJ (1981) Genetic analysis of the RecE pathway of genetic recombination in Escherichia coli K-12. J Bacteriol 145:521–532
Gowrishankar J (1985) Identification of osmoresponsive genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J Bacteriol 164:434–445
Gowrishankar J, Jayashree P, Rajkumari K (1986) Molecular cloning of an osmoregulatory locus in Escherichia coli: increased proU gene dosage results in enhanced osmotolerance. J Bacteriol 168:1197–1204
Guerola N, Ingraham JL, Cerda-Olmedo E (1971) Induction of closely linked multiple mutations by nitrosoguanidine. Nature New Biol 230:122–125
Hall SD, Kane MF, Kolodner RD (1993) Identification and characterization of the Escherichia coli RecT protein, a protein encoded by the recE region that promotes renaturation of homologous single-stranded DNA. J Bacteriol 175:277–287 (erratum, J Bacteriol 1993, 175:1211)
Harris RS, Longerich S, Rosenberg SM (1994) Recombination in adaptive mutation. Science 264:258–260
Horii ZI, Clark AJ (1973) Genetic analysis of the RecF pathway of genetic recombination in Escherichia coli K-12: isolation and characterization of mutants. J Mol Biol 80:327–344
Jayashree P (1993) Genetics of osmoregulation in Escherichia coli: isolation and characterization of mutants altered in regulation of the proU operon. PhD Thesis, Jawaharlal Nehru University, New Delhi, India
Kleckner N, Barker DF, Ross DG, Botstein D (1978) Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics 90:427–450
Kolodner R, Fishel RA, Howard M (1985) Genetic recombination of bacterial plasmid DNA: effect of RecF pathway mutations on plasmid recombination in Escherichia coli. J Bacteriol 163:1060–1066
Kolodner R, Hall SD, Luisi-DeLuca C (1994) Homologous pairing proteins encoded by the Escherichia coli recE and recT genes. Mol Microbiol 11:23–30
Kusano K, Nakayama K, Nakayama H (1989) Plasmid-mediated lethality and plasmid multimer formation in an Escherichia coli recBC sbcBC mutant. Involvement of RecF recombination pathway genes. J Mol Biol 209:623–634
Kusano K, Sunohara Y, Takahashi N, Yoshikura H, Kobayashi I (1994) DNA double-strand break repair: genetic determinants of flanking crossing-over. Proc Natl Acad Sci USA 91:1173–1177
Kushner SR, Nagaishi H, Templin A, Clark AJ (1971) Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci USA 68:824–827
Kushner SR, Nagaishi H, Clark AJ (1972) Indirect suppression of recBC recC mutations by exonuclease I deficiency. Proc Natl Acad Sci USA 69:1366–1370
Kushner SR, Nagaishi H, Clark AJ (1974) Isolation of exonuclease VIII: the enzyme associated with the bcA indirect suppressor. Proc Natl Acad Sci USA 71:3593–3597
Lloyd RG, Buckman C (1985) Identification and genetic analysis of sbcC mutations in commonly used recBC sbcB strains of Escherichia coli K-12. J Bacteriol 164:836–844
Lloyd RG, Buckman C (1991) Genetic analysis of the ecG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J Bacteriol 173:1004–1011
Luisi-DeLuca C, Lovett ST, Kolodner RD (1989) Genetic and physical analysis of plasmid recombination in recG recC sbcB and recB recC sbcA Escherichia coli K-12 mutants. Genetics 122:269–278
Mahajan SK (1988) Pathways of homologous recombination in Escherichia coli. In: Kucherlapati R, Smith G (eds) Genetic recombination. American Society of Microbiology, Washington DC, pp 87–140
Mahajan SK, Chu CC, Willis DK, Templin A, Clark AJ (1990) Physical analysis of spontaneous and mutagen-induced mutants of Escherichia coli K-12 expressing DNA exonuclease VIII activity. Genetics 125:261–273
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Niki H, Ogura T, Hiraga S (1990) Linear multimer formation of plasmid DNA in Escherichia coli hopE (recD) mutants. Mol Gen Genet 244:1–9
Patient ME, Summers DK (1993) ColEl multimer formation triggers inhibition of Escherichia coli cell division. Mol Microbiol 9:1089–1095
Phillips GJ, Prasher DC, Kushner SR (1988) Physical and biochemical characterization of cloned sbcB and xonA mutations from Escherichia coli K-12. J Bacteriol 170:2089–2094
Picksley SM, Lloyd RG, Bucman C (1984) Genetic analysis and regulation of inducible recombination in Escherichia coli K-12. Cold Spring Harbor Symp Quant Biol 49:469–474
Sandigursky M, Franklin WA (1992) DNA deoxyribophosphodiesterase of Escherichia coli is associated with exonuclease I. Nucleic Acids Res 20:4699–4703
Sawitzke JA, Stahl FW (1992) Phage lambda has an analog of Escherichia coli rec0, recR and recF genes. Genetics 130:7–16
Smith GR (1988) Homologous recombination in procaryotes. Microbiol Rev 52:1–28
Templin A, Kushner SR, Clark AJ (1972) Genetic analysis of mutations indirectly suppressing recB and reC mutations. Genetics 72:205–215
Walker GC (1984) Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev 48:60–93
Wang T-CV, Smith KC (1985) Mechanism of sbcB-suppression of the recBC-deficiency in postreplication repair in UV-irradiated Escherichia coli K-12. Mol Gen Genet 201:186–191
West SC (1992) Enzymes and molecular mechanisms of genetic recombination. Annu Rev Biochem 61:603–640
Willetts NS, Clark AJ (1969) Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol 100:231–239
Yajko EM, Valentine MC, Weiss B (1974) Mutants of Escherichia coli with altered deoxyribonculeases. II. Isolation and characterization of mutants for exonuclease 1. J Mol Biol 85:223–243
Author information
Authors and Affiliations
Additional information
Communicated by R. Devoret
Rights and permissions
About this article
Cite this article
Jayashree, P., Gowrishankar, J. A new phenotype for shcB mutations in Escherichia coli: RecA-dependent increase in plasmid-borne gene expression. Molec. Gen. Genet. 246, 648–656 (1995). https://doi.org/10.1007/BF00298972
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00298972