Summary
In domestic and wild pigs motor nuclei of the M. semitendinosus were labelled by retrograde transport of horseradish peroxidase (HRP) from muscle to spinal cord. Further motoneurons in the lumbosacral cord were stained with Luxol-fast-blue and cresyl violet.
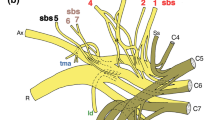
Motoneurons innervating M. semitendinosus were present in the ventral horns of the last (6th) lumbal and the first sacral cord segments. They were localized within two motor columns lying parallel to each other in the medial and lateroventral position. Both parts of the semitendinosus motor nuclei showed a spindle-like shape with both cranial and caudal enlargements.
Localization, extent, and shape of the semitendinosus motor nuclei were similar in domestic and wild pigs. The average motoneuron diameter was larger in domestic than in wild pigs. The lumbosacral cord of the wild pig was thicker than that of the domestic pig.
It is suggested that the size of α-motoneurons has increased as a result of selective breeding after domestication. This process might correlate with a higher incidence of myopathies in domestic pigs.
Similar content being viewed by others
References
Beermann DH, Cassens RG (1977) Terminal innervation ratios and fiber type grouping in normal porcine skeletal muscle. Anat Embryol 150:123–127
Bickhardt K, Chevalier H-J, Giese W, Reinhardt HJ (Eds) (1972) Akute Rückenmuskelnekrose und Belastungsmyopathie beim Schwein. Fortschritte der Vet. Med. Berlin/Hamburg, Parey
Buller AJ, Eccles JC, Eccles RM (1960a) Differentiation of fast and slow muscles in the cat hind limb. J Physiol 150:399–416
Buller AJ, Eccles JC, Eccles RM (1960b) Interactions between motoneurones and muscles in respect of the characteristic speed of their responses. J Physiol 150:417–439
Burke RE, Strick PL, Kanda K, Kim CC, Walmsley B (1977) Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol 40:667–680
Cassens RG, Cooper CC, Briskey EJ (1969) The occurrence and histochemical characterization of giant fibers in the muscle of growing and adult animals. Acta Neuropathol 12:300–308
Drommer W (1972) Permeabilitätsstörungen im zentralen Nervensystem des Schweines. Acta Neuropathol 20:299–315
Henneman E (1981) Recruitment of motoneurons: the size principle. Progr Clin Neurophysiol 9:26–60
Henneman E, Somjen G, Carpenter DO (1965a) Functional significance of cell size in spinal motoneurons. J Neurophysiol 28:560–580
Henneman E, Somjen G, Carpenter DO (1965b) Excitability and inhibitibility of motoneurons of different sizes. J Neurophysiol 28:599–620
Janković ZK (1954) Das Rückenmark, die Rückenmarkshüllen, ihre Zwischenräume und ihre Topographie bei den Schweinen. Acta Vet (Beograd) 4:73–88
Johannsen U, Kunz G (1980) Untersuchungen zur Pathomorphologie der Skelettmuskulatur bei Schweinen mit Transporttod. Arch Exp Vet Med 34:273–289
Kluever H, Barrera E (1953) A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol 12:400–403 (1953)
Luescher HR, Ruenzel P, Henneman E (1979) How the size of motoneurons determines their susceptibility to discharge. Nature 289:859–861
Martin AH, Fredeen HT, L'Hirondelle PJ, Murray AC, Weiss GM (1981) Pork quality attributes: Their estimation and their relationship with carcass composition of commercial pigs. Can J Anim Sci 61:289–298
McHanwell S, Biscoe TJ (1981) The sizes of motoneurons supplying hindlimb muscles in the mouse. Proc R Soc Lond B 213:201–216
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: A non-carcinogenic blue reactionproduct with superior sensitivity for visualizing afferents and efferents. J Histochem Cytochem 26:106–117
Nickel R, Schummer A, Seiferle E (eds) (1975) Lehrbuch der Anatomie der Haustiere. Bd. IV: Nervensystem, endokrine Drüsen, Sinnesorgane. P. Parey, Berlin und Hamburg
Pelckmans A (1964) The influence of fixation upon staining according to Kluever and Barrera. Acta Histochem 19:329–336
Rapoport S (1978) Location of sternocleidomastoid and trapezius motoneurons in the cat. Brain Res 156:339–344
Rexed B (1952) The cytoarchitectonic organization of the spinal cord in the rat. J Comp Neurol 96:415–495
Rexed B (1964) Some aspects of the cytoarchitectonics and synaptology of the spinal cord. In: Eccles JC, Schadé JP (Eds): Organization of the spinal cord. Progr Brain Res 11:58–92
Romanes GJ (1964) The motor pools of the spinal cord. In: Eccles JC, Schadé JP (Eds) Organization of the spinal cord. Progr Brain Res 11:93–119
Salmons S, Sréter FA (1976) Significance of impulse activity in the transformation of skeletal muscle type. Nature 263:30–34
Salmons S, Vrbová G (1969) The influence of activity on some contractile characteristics of mammalian fast and slow muscle. J Physiol 201:535–549
Swatland HJ, Cassens RG (1973) Prenatal development, histochemistry and innervation of porcine muscle. J Anim Sci 36:343–354
Szentkuti L, Cassens RG (1979) Motor innervation of myofiber types in porcine skeletal muscle. J Animal Sci 49:693–700
Szentkuti L, Niemeyer B, Schlegel O (1981) Vergleichende Untersuchung von Muskelfasertypen mit der Myosin-ATPase-Reaktion im M. longissimus dorsi von Haus-und Wildschweinen. Dtsch Tierärztl Wschr 88:407–411
Webber CL Jr (1979) The structural and functional ofganization of the phrenic motoneuron pool. Am Rev Resp Dis 119:57–60
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft
Rights and permissions
About this article
Cite this article
Szenkuti, L., Bruns, J. Motoneurons of M. semitendinosus in domestic and wild pigs. Anat Embryol 167, 213–228 (1983). https://doi.org/10.1007/BF00298512
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00298512