Summary
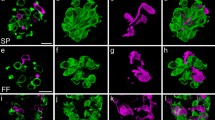
Keratin filaments of epithelial- and taste-bud cells in the circumvallate papillae of adult and developing mice were studied by immunocytochemistry using monoclonal antikeratin antibodies (PKK2 and PKK3) and by conventional electron microscopy. Elongated cells (type-I,-II, and-III cells) of the taste buds were stained by PKK3 antibody, which reacts with 45-kdalton keratin, whereas basal cells of the taste buds and surrounding epithelial cells showed negative staining with PKK3. Such PKK3-reactive cells occurred at 0 day after birth, when taste-buds first appeared in the dorsal surface epithelium of the papillae. Thus 45-kdalton keratin seems to be an excellent immunocytochemical marker for identifying taste-bud cells. Epithelial cells in all layers of the trench wall and basal layer cells of the dorsal surface contained densely aggregated bundles of keratin filaments that reacted with PKK2 antibody, but not with PKK3. In contrast, taste-bud cells and spinous and granular layer cells of the dorsal surface possessed loose aggregated bundles of filaments that reacted with PKK3, but not with PKK2. These results suggest that the aggregation and distribution pattern of keratin filaments may reflect differences in the keratin subtypes that comprise these filaments.
Similar content being viewed by others
References
Appleton J, Tyldesley WR (1971) Observations on the ultrastructure of the buccal epithelium of the rat. Arch Oral Biol 16:1071–1088
Banks-Schlegel SP (1982) Keratin alterations during embryonic epidermal differentiation: a presage of adult epidermal maturation. J Cell Biol 93:551–559
Brody I (1960) The ultrastructure of the tonofibrils in the keratinization process of normal human epidermis. J Ultrastruct Res 4:264–297
Dale BA, Resing KA, Lonsdale-Eccles JD (1985) Filaggrin: a keratin filament associated protein. Ann NY Acad Sci 455:330–342
Denk H, Krepler R, Lackinger E, Artlieb U, Franke WW (1982) Biochemical and immunocytochemical analysis of the intermediate filament cytoskeleton in human hepatocellular carcinomas and in hepatic neoplastic nodules of mice. Lab Invest 46:584–596
Eichner R, Bonitz P, Sun T (1984) Classification of epidermal keratins according to their immunoreactivity, isoelectric point and mode of expression. J Cell Biol 98:1388–1396
Eichner R, Rew P, Engel A, Aebi U (1985) Human epidermal keratin filaments: studies on their structure and assembly. Ann NY Acad Sci 455:381–402
Eichner R, Sun T-T, Aebi U (1986) The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol 102:1767–1777
Franke WW, Weber K, Osborn M, Schmid E, Freudenstein C (1978) Antibody to prekeratin: decoration of tonofilament-like arrays in various cells of epithelial character. Exp Cell Res 116:429–445
Franke WW, Schiller DL, Moll R, Winter S, Schmid E, Engelbrecht I, Denk H, Krepler R, Platzer B (1981) Diversity of cytokeratins: differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol 153:933–959
Fuchs E, Hanukoglu I, Marchuk D, Grace MP, Kim KH (1985) The nature and significance of differential keratin gene expression. Ann NY Acad Sci 455:436–450
Fujimoto S, Murray RG (1970) Fine structure of degeneration and regeneration in denervated rabbit vallate taste buds. Anat Rec 168:393–414
Gustafsson H, Kjorell U, Eriksson A, Virtanen I, Thornell LE (1988) Distribution of intermediate filament proteins in developing and adult salivary glands in man. Anat Embryol 178:243–251
Hayward AF, Hamilton AI, Hackermann MMA (1973) Histological and ultrastructural observations on the keratinizing epithelia of the palate of the rat. Arch Oral Biol 18:1041–1057
Lane EB (1982) Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol 92:665–673
Lane EB, Bartek J, Purkis PE, Leigh IM (1985) Keratin antigens in differentiating skin. Ann NY Acad Sci 455:241–258
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Moll R, Moll I, Franke WW (1984) Identification of Merkel cells in human skin by specific cytokeratin antibodies. Differentiation 28:136–154
Murray RG (1973) The ultrastructure of taste buds. In: Friedman I (ed) The ultrastructure of sensory organs. North-Holland, Amsterdam London, and American Elsevier, New York, pp 1–81
Odajima T, Kaku T (1978) Ultrastructural study on experimental lingual carcinogenesis in the hamster. I. Ultrastructural study of normal tongue epithelium with special reference to the process of keratinization. Sapporo Med J 47:225–243
Quinlan RA, Schiller DL, Hatzfeld M, Achtstatter T, Moll R, Jorcano JL, Magin TM, Franke WW (1985) Patterns of expression and organization of cytokeratin intermediate filaments. Ann NY Acad Sci 455:282–306
Rentrop M, Knapp B, Winter H, Schweizer J (1986) Differential localization of distinct keratin mRNA-species in mouse tongue epithelium by in situ hybridization with specific cDNA probes. J Cell Biol 103:2583–2591
Savino W, Dardenne M (1988) Immunohistochemical studies on a human thymic epithelial cell subset defined by the anti-cytokeratin 18 monoclonal antibody. Cell Tissue Res 254:225–231
Schlegel R, Banks-Schlegel S, Pinkus GS (1980) Immunocytochemical localization of keratin in normal human tissues. Lab Invest 42:91–96
Skerrow D, Skerrow CJ (1983) Tonofilament differentiation in human epidermis, isolation and polypeptide chain composition of keratinocyte subpopulations. Exp Cell Res 143:27–35
Steinert PM, Idler WW, Zhou XM, Johnson LD, Parry DAD, Steven AC, Roop DR (1985) Structural and functional implications of amino acid sequences of keratin intermediate filament subunits. Ann NY Acad Sci 455:451–461
Sun TT, Shih C, Green H (1979) Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci USA 76:2813–2817
Sun TT, Eichner R, Nelson WG, Tseng SCG (1983) Keratin classes: molecular markers for different types of epithelial differentiation. J Invest Derm 81:109s-115s
Sun TT, Tseng SCG, Huang AJW, Cooper D, Eichner R (1985) Monoclonal antibody studies of mammalian epithelial keratins: a review. Ann NY Acad Sci 455:307–329
Takeda M, Hoshino T (1975) Fine structure of taste buds in the rat. Arch Histol Jpn 37:395–413
Takeda M, Suzuki Y, Shishido Y (1985) Effects of colchicine on the ultrastructure of mouse taste buds. Cell Tissue Res 242:409–416
Takeda M, Obara N, Suzuki Y (1988) Intermediate filaments in mouse taste bud cells. Arch Histol Cytol 51:99–108
Tseng SCG, Javinen MJ, Nelson WG, Huang JW, Woodcock-Mitchell J, Sun TT (1982) Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody study. Cell 30:361–372
Woodcock-Mitchell J, Eichner R, Nelson WG, Sun T (1982) Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol 95:580–588
Zelickson AS, Hartmann JF (1962) An electron microscope study of normal human nonkeratinizing oral mucosa. J Invest Derm 38:99–106
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takeda, M., Obara, N. & Suzuki, Y. Keratin filaments of epithelial and taste-bud cells in the circumvallate papillae of adult and developing mice. Cell Tissue Res 260, 41–48 (1990). https://doi.org/10.1007/BF00297488
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00297488