Summary
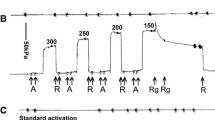
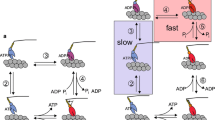
To probe the substrate requirements for the actomyosin chemomechanical interaction, the effects of a series of eight new non-nucleotide ATP analogues on actomyosin-catalysed hydrolysis rates and on fibre mechanics have been investigated. These analogues have substitutions of new functional groups at the 2- and 4- positions of the ATP analogues, 2-[(4-azido-2-nitrophenyl)amino]ethyl triphosphate (NANTP), and 3-[(4-nitrophenyl)amino]propyl triphosphate (PrNANTP). Previous work has shown NANTP but not PrNANTP will support active tension and shortening in skinned muscle fibres in a manner almost identical to ATP. Here all 2- and 4- phenyl substituted analogues had myosin subfragment 1 (S1) NTPase hydrolysis rates higher than ATP and the rates were stimulated by addition of actin. In general, the replacement of the 4-azido group of NANTP with-H,-NO2 or-NH2 had small effects on fibre mechanics while replacement of 2-NO2 group with-H or-NH2 dramatically lowered the ability of the new analogues to support active tension and shortening. All PrNANTP-based analogues were ineffective in supporting active tension or shortening. We found no correlation between S1 or actoS1 NTPase rates and any mechanical parameters. However, for all analogues there was a strong correlation between the maximal velocity of shortening (V max) and isometric tension (P o). A three-state, chemomechanical model is proposed in which the analogues effect the transition rate into a strongly-bound, force-producing crossbridge state to account for this correlation. These studies identify 2-[(2-nitrophenyl)amino]ethyl triposphate as the chemically simplest ATP analogue which closely mimics the effect of ATP in skinned muscle fibres.
Similar content being viewed by others
References
ÁGAI, B., DOLESCHALL, G., HORNYAK, GY., LEMPERT, K. & SIMIG, GY. (1975) Condensed 1,3,5-triazepines-III. Derivatives of 4,5-dihydro-[1,3,5]triazepino[1,2-a]benzimidazole. Tetrahedron 32, 839–42.
BARANY, M. (1967) ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 50, 197–216 (Suppl.).
BRENNER, B. (1990) Muscle mechanics and biochemical kinetics. In: Molecular Mechanisms in Muscular Contraction (edited by SQUIRE, J. M.) pp. 77–149. London: Macmillan Press.
CHASE, P. B. & KUSHMERICK, M. J. (1988) Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys. J. 53, 935–46.
COLE, D. G. & YOUNT, R. G. (1990) Photolabeling of the 6S and 10S conformations of gizzard myosin with 3′(2′)-O-(4-benzoyl)benzoyl-ATP. J. Biol. Chem. 265, 22537–46.
COOKE, R. (1986) The mechanism of muscle contraction. CRC Crit. Rev. Biochem. 21, 53–118.
COOKE, R., FRANKS, K., LUCIANNI, G. & Pate, E. (1988) The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J. Physiol. (Lond.) 395, 77–99.
DANTZIG, J. A., GOLDMAN, Y. E., LACKTIS, J., MILLAR, N. C. & HOMSHER, E. (1992) Reversal of the cross-bridge force generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol. 451, 247–78.
ECCLESTON, J. F. & TRENTHAM, D. R. (1979) Magnesium ion dependent rabbit skeletal muscle myosin guanosine and thioguanosine triphosphatase mechanism and a novel guanosine diphosphatase reaction. Biochemistry 18, 2896–904.
EISENBERG, E., HILL, T. L. & CHEN, Y. (1980) Cross-bridge model of muscle contraction. Biophys. J. 29, 195–227.
FERSHT, A. R., SHI, J.-P., KNILL-JONES, J., LOWE, D.M., WILKINSON, A. J., BLOW, D. M., BRICK, P., CARTER, P., WAYE, M. M. Y. & WINTER, G. (1985) Hydrogen bonding and biological specificity analysed by protein engineering. Nature 314, 235–8.
GARABEDIAN, T. & YOUNT, R.G. (1990) Direct photolabeling of gizzard myosin with UDP and vanadate places Glu-185 of the heavy chain at the active site. J. Biol. Chem. 265, 22547–53.
HIBBERD, M. G. & TRENTHAM, D. R. (1986) Relationships between chemical and mechanical events during muscular contraction. Ann. Rev. Biophys. Biophys. Chem. 15, 119–61.
HIGHSMITH, S. (1976) Interaction of the actin and nucleotide binding sites on myosin subfragment-1. J. Biol. Chem. 251, 6170–2.
HILL, A. V. (1938) The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B. Biol. Sci. 126, 136–95.
HUXLEY, A.F.. (1957) Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255–318.
LANZETTA, P. A., ALVAREZ, L. J., REINACH, D. S. & CANDIA, O. A. (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem 100, 95–7.
MAHMOOD, R., ELZINGA, M. & YOUNT, R. G. (1989) Serine-324 of myosin's heavy chain is photoaffinity-labeled by 3′(2′)-O-(4-benzoyl)-benzoyladenosine triphosphate. Biochemistry 28, 3989–95.
MOLNAR, J. & LOBNARD, L. (1961) Studies on apyrases. Arch. Biochem. Biophys. 93, 353–63.
NAKAMAYE, K. L., WELLS, J. A., BRINDENBAUGH, R. L., OKAMOTO, Y. & YOUNT, R. G. (1985) 2-[(4-azido-2-nitrophenyl)amino]-ethyltriphosphate, a novel chromophoric and photoaffinity analogue of ATP. Synthesis, characterization and interaction with myosin subfragment 1. Biochemistry 24, 5226–35.
OKAMOTO, Y. & YOUNT, R. G. (1985) Identification of an active site peptide of skeletal myosin after photoaffinity labeling with N-(4-azido-2-nitrophenyl)-2-aminoethyl diphosphate. Proc. Natl. Acad. Sci. USA. 82, 1575–9.
PANUNTO, T. W., URBÁNCZYK-LIPKOWSKA, Z., JOHNSON, R. & Etter, M. C. (1987) Hydrogen-bond formation in nitroanilines: the first step in designing acentric materials. J. Am. Chem. Soc. 109, 7786–97.
PARDEE, J. D. & SPUDICH, J. A. (1986) Purification of muscle myosin. Method Enzymol. 85, 164–81.
PATE, E. & COOKE, R. (1989) A model of cross-bridge action, the effects of MgATP, ADP and Pi. J. Muscle Res. Cell Motil. 10, 181–96.
PATE, E., NAKAMAYE, K. L., FRANKS-SKIBA, K., YOUNT, R. G. & COOKE, R. (1991) Mechanics of glycerinated muscle fibers using non-nucleoside triphosphate substrates. Biophys. J. 59, 598–605.
PATE, E., FRANKS, K., WHITE, H. D. & COOKE, R. (1993) The use of differing nucleotides to investigate cross-bridge kinetics. J. Biol. Chem. 286, 10046–53.
PUTNINS, R. F. & YAMADA, E. W. (1975) Colorimetric determination of inorganic pyrophosphate by a manual or automated method. Anal. Biochem. 68, 185–95.
RAYMENT, I. & WINKELMANN, D. A. (1984) Crystallization of myosin subfragment 1. Proc. Natl. Acad. Sci. USA 81, 4378–80.
ROSENFELD, S. S. & TAYLOR, E. W. (1987) The mechanism of regulation of actomyosin subfragment 1 ATPase. J. Biol. Chem. 264, 9984–93.
SCHOENBERG, M. (1988) Characterization of the myosin adenosine triphosphate (M.ATP) cross-bridge in rabbit and frog skeletal muscle fibers. Biophys. J. 54, 135–48.
SHIBATA-SEKIYA, K. (1973) ATP analogues. In Muscle Proteins, Muscle Contraction and Cation Transport (edited by TONOMURA, Y.), pp. 257–271. Baltimore: University Park Press.
STAROS, J. V., BAYLEY, H., STANDRING, D. N. & KNOWLES, J. R. (1978) Reduction of aryl azides by thiols, implication for the use of photoaffinity reagents. Biochem. Biophys. Res. Commun. 80, 568–72.
WELLS, J. A., WERBER, M. M., LEGG, J. I. & YOUNT, R. G. (1979) Inactivation of myosin subfragment one by Cobalt(II)/Cobalt(III) phenanthroline complexes. 1. Incorporation of Co(III) by in situ oxidation of Co(II). Biochemistry 18, 4793–6.
WHITE, H. D., BELKNAP, B. & JIANG, W. (1993) Kinetics of binding and hydrolysis of a series of nucleoside triphosphates by actomyosin-S1: relationship between solution rate constants and properties of muscle fibres. J. Biol. Chem. 268, 10039–45.
WOLEDGE, R. C., CURTIN, N. A. & HOMSHER, E. (1985) Energetic Aspects of Muscle Contraction. London: Academic Press.
YOUNT, R. G., BABCOCK, D., BALLANTYNE, W. & OJALA, D. (1971) Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P-N-P linkage. Biochemistry 10, 2484–9.
YOUNT, R. G., CREMO, C. R., GRAMMER, J. C. & KERWIN, B. A. (1992) Photochemical mapping of the active site of myosin. Phil. Trans. R. Soc. Lond. B 336, 55–61.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, D., Pate, E., Cooke, R. et al. Synthesis of non-nucleotide ATP analogues and characterization of their chemomechanical interaction with muscle fibres. J Muscle Res Cell Motil 14, 484–497 (1993). https://doi.org/10.1007/BF00297211
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00297211