Summary
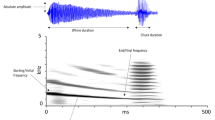
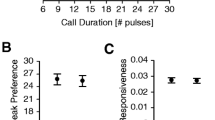
Variation in seasonal mating success among male natterjack toads (Bufo calamita) was influenced by the number of nights that males spent at the pond and by male body size. Large males produced louder and lower frequency calls than small males, and maintained larger acoustic territories. After arriving at the pond, one half of all observed females mated with the first male they encountered. The remainder visited several males before initiating amplexus, but no criteria could be identified that females might have used when deciding whether to accept or reject a male. Movements between several males seem to be best explained by low female responsiveness to male advertisement calls on cold nights which were nonoptimal for oviposition. Females attempted to reject non-calling males both before and after amplexus, but this may be a mechanism to avoid mismating with males of the common toad (Bufo bufo), an explosive breeder that utilised the same pond. In two-choice playback experiments using synthetic advertisement calls, females showed no preference for calls based on their frequency. Females preferred calls of intermediate pulse repetition rate equivalent to those produced by a male at the same body temperature. Pulse rate is thus potentially subject to stabilishing selection and may be an important character for species-recognition. Females preferred fast rather than slow call rates, but only when the alternative rates were extreme. They also preferred calls which they perceived at the highest sound pressure level, but did not discriminate between absolute sound pressure levels of alternative stimuli at different distances. Since females that delay mating and oviposition may suffer predation, it is suggested that female preference for loud, rapidly repeated calls may be adaptive in the sense of minimizing the costs of locating conspecific males, rather than maximizing the probability of obtaining a high quality mate. Competition between males to maintain large acoustic territories and produce calls that can be easily detected by females would seem to be a sufficient mechanism to explain the evolution of the striking calls produced by male natterjacks.
Similar content being viewed by others
References
Arak A (1983a) Male-male competition and mate choice in anuran amphibians. In: Bateson P (ed) Mate choice. Cambridge Univ Press, Cambridge, pp 181–210
Arak A (1983b) Sexual selection through male-male competition in natteriack toad choruses. Nature 306:261–262
Arak A (1988) Callers and satellites in the natterjack toad: evolutionarily stable decision rules. Anim Behav 36:416–432
Bradbury JW (1981) The evolution of leks. In: Alexander RD, Tinkle DW (eds) Natural selection and social behavior: recent research and new theory. Chiron Press, New York, pp 138–169
Brown L (1981) Patterns of female choice in mottled sculpins (Cottidae: Teleostei). Anim Behav 29:375–382
Crews D (1975) Effects of different components of male courtship behaviour on environmentally induced ovarian recrudescence and mating preferences in the lizard, Anolis carolinensis. Anim Behav 23:349–356
Davies NB, Halliday TR (1979) Competitive mate searching in male common toads Bufo bufo. Anim Behav 27:1253–1267
Doherty JA, Gerhardt HC (1984) Evolutionary and neurobiological implications of selective phonotaxis in the spring peeper (Hyla crucifer). Anim Behav 32:875–881
Emlen ST, Oring LW (1977) Ecology, sexual selection and the evolution of mating systems. Science 197:215–223
Fellers GM (1979) Aggression, terrioriality and mating behaviour in North American treefrogs. Anim Behav 27:107–119
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford
Forester DC, Czarnowsky R (1985) Sexual selection in the spring peeper, Hyla crucifer (Amphibia, Anura): role of the advertisement call. Behaviour 92:112–128
Gerhardt HC (1978) Temperature coupling in the vocal communication system of the gray treefrog, Hyla versicolor. Science 199:992–994
Gerhardt HC (1982) Sound pattern recognition in some North American treefrogs (Anura: Hylidae): implications for mate choice. Amer Zool 22:581–595
Halliday T (1983) Do frogs and toads choose their mates? Nature 306:226–227
Hinde RA, Steel E (1978) The influence of daylength and male vocalizations on the oestrogen-dependent behavior of female canaries and budgerigars. Adv Study Behav 8:40–74
Howard RD (1978) The evolution of mating strategies in bullfrogs, Rana catesheiana. Evolution 32:850–871
Howard RD (1981a) Male age-size distribution and male mating success in bullfrogs. In: Alexander RD, Tinkle DW (eds) Natural selection and social behaviour: recent research and new theory. Chiron Press, New York, pp 61–77
Howard RD (1981b) Sexual dimorphism in bullfrogs. Ecology 62:303–310
Howard RD, Kluge AG (1985) Proximate mechanisms of sexual selection in wood frogs. Evolution 39:260–277
Janetos AC (1980) Strategies of mate choice: a theoretical analysis. Behav Ecol Sociobiol 7:107–112
Kirkpatrick M (1982) Sexual selection and the evolution of female choice. Evolution 36:1–12
Kluge AG (1981) The life history, social organization, and parental behaviour of Hyla rosenbergi Boulenger, a nestbuilding gladiator frog. Misc Publs Mus Zool Univ Michigan 160:1–170
Klump GM, Gerhardt HC (1987) Use of non-arbitrary acoustic criteria in mate choice by female gray tree frogs. Nature 326:286–288
Lande R (1981) Models of speciation by sexual selection of polygenic traits. Proc Natl Acad Sci USA 78:3721–3725
McFarland D (1985) Animal behaviour. Pitman Press, Bath
Majerus MEN, O'Donald P, Weir J (1982) Female mating preference is genetics. Nature 300:521–522
Mathias JH (1971) The comparative ecologies of two species of Amphibia (Bufo bufo and Bufo calamita) on the Ainsdale Sand Dunes National Nature Reserve. PhD thesis, University of Manchester
O'Donald P (1980) Genetic models of sexual selection. Cambridge Univ Press, Cambridge
Olson DH, Blaustein AR, O'Hara RK (1986) Mating pattern variability among western toad (Bufo boreas) populations. Oecologia 70:351–356
Parker GA (1982) Phenotype limited evolutionarily stable strategies. In: King's College Sociobiology Group eds) Current problems in sociobiology. Cambridge Univ Press, Cambridge, pp 173–201
Parker GA (1983) Mate quality and mating decisions. In: Bateson P (ed) Mate choice. Cambridge Univ Press, Cambridge, pp 141–166
Robertson JGM (1984) Acoustic spacing by breeding males of Uperoleia rugosa (Anura: Leptodactylidae). Z Tierpsychol 64:283–297
Robertson JGM (1986) Female choice, male strategies and the role of vocalizations in the Australian frog Uperoleia rugosa. Anim Behav 34:773–784
Rose GJ, Capranica RR (1984) Processing amplitude-modulated sounds by the auditory midbrain of two species of toads: matched temporal filters. J Comp Physiol A 154:211–219
Ryan MJ (1985) The túngara frog. Univ of Chicago Press, Chicago
Salthe SW, Mecham JS (1974) Reproductive courtship patterns. In: Lofts B (ed) Physiology of the Amphibia, vol 2. Academic Press, New York, pp 209–251
Schwartz JJ (1986) Male calling behavior and female choice in the neotropical treefrog Hyla microcephala. Ethology 73:116–127
Searcy WA, Andersson M (1986) Sexual selection and the evolution of song. Ann Rev Ecol Syst 17:507–533
Sullivan BK (1982a) Sexual selection in Woodhouse's toad (Bufo woodhousei). I. Chorus organization. Anim Behav 30:680–686
Sullivan BK (1982b) Male mating behaviour in the Great Plains toad (Bufo cognatus). Anim Behav 30:939–940
Sullivan BK (1983) Sexual selection in Woodhouse's toad (Bufo woodhousei). II. Female choice. Anim Behav 31:1011–1017
Wells KD (1977) Territoriality and male mating success in the green frog (Rana clamitans). Ecology 58:750–762
Wells KD, Schwartz JJ (1984) Vocal communication in a neotropical treefrog, Hyla ebraccata: advertisement calls. Anim Behav 32:405–420
Whitney CL, Krebs JR (1975) Mate selection in Pacific tree frogs, Hyla regilla. Nature 255:325–326
Wittenberger JF (1983) Tactics of mate choice. In: Bateson P (ed) Mate choice. Cambridge Univ Press, Cambridge, pp 435–447
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arak, A. Female mate selection in the natterjack toad: active choice or passive atraction?. Behav Ecol Sociobiol 22, 317–327 (1988). https://doi.org/10.1007/BF00295100
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00295100