Abstract
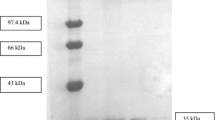
The extracellular lipase of Staphylococcus warneri was secreted as a protein with an apparent molecular mass of 90 kDa. It was then sequentially processed in the supernatant to a protein of 45 kDa. Tryptic digestion of the crude extract resulted in a homogeneous sample containing only the 45-kDa form. Purification was achieved by hydrophobic chromatography. Purified lipase had an optimum pH of 9.0 and an optimum temperature of 25°C. The enzyme was stable within the range pH 5.0–9.0; it had a broad substrate specificity. The results of inhibition studies were consistent with the view that lipases possess a serine residue at the catalytic site.
Similar content being viewed by others
Literature Cited
Berdagué JL, Monteil P, Montel MC, Talon R (1993) Effects of starter cultures on the formation of flavour compounds in dry sausage. Meat Sci 35:275–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carrere J, Serre G, Vincent C, Croute F, Soleilhavoup JP, Thouvenot JP, Figarella C (1987) Human serum pancreatic lipase and trypsin 1 in aging: enzymatic and immunoenzymatic assays. J Gerontol 42:315–317
Farrell AM, Foster TJ, Holland KT (1993) Molecular analysis and expression of the lipase of Staphylococcus epidermidis. J Gen Microbiol 139:267–277
Götz F, Popp F, Korn E, Schleifer KH (1985) Complete nucleotide sequence of the lipase from Staphylococcus hyicus in Staphylococcus carnosus. Nucleic Acids Res 13:5895–5906
Hedström SA, Nillsson-Ehle P (1983) Trioleoylglycerol lipolysis by Staphylococcus aureus strains from recurrent furunculosis, pyomyositis, impetigo and osteomyelitis. Acta Pathol Microbiol Immunol Scand Sect B91:169–173
Jäger S, Demleitner G, Götz F (1992) Lipase of Staphylococcus hyicus: analysis of the catalytic triad by site-directed mutagenesis. FEMS Microbiol Lett 100:249–254
Jürgens D, Huser H (1981) Large-scale purification of staphylococcal lipase by hydrophobic interaction chromatography. J Chromatogr 216:295–301
Jürgens D, Huser H, Brunner H, Fehrenbach FJ (1981) Purification and characterization of Staphylococcus aureus lipase. FEMS Microbiol Lett 12:195–199
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lechner M, Märkl H, Götz F (1988) Lipase production of Staphylococcus carnosus in a dialysis fermentor. Appl Microbiol Biotechnol 28:345–349
Montel MC, Talon R, Cantonnet M, Fournaud J (1992) Identification of Staphylococcus from French dry sausage. Lett Appl Microbiol 15:73–77
Montel MC, Talon R, Berdagué JL, Cantonnet M (1993) Effects of starter cultures on the biochemical characteristics of French dry sausages. Meat Sci 35:229–240
Muraoka T, Ando T, Okudo H (1982) Purification and properties of a novel lipase from Staphylococcus aureus 226. J Biochem 92:1933–1939
O'Farrell PH (1975) High resolution two dimensional electrophoresis of proteins. J Biol Chem 25:4007–4021
Rollof J, Normark S (1992) In vivo processing of Staphylococcus aureus lipase. J Bacteriol 174:1844–1847
Rollof J, Hedström SA, Nillsson-Ehle P (1987a) Purification and characterization of lipase from Staphylococcus aureus. Biochim Biophys Acta 921:364–369
Rollof J, Hedström SA, Nillsson-Ehle P (1987b) Positional specificity and substrate preference of purified Staphylococcus aureus lipase. Biochim Biophys Acta 921:370–377
Sarath G, De la Motte RS, Wagner FW (1989) Protease assay methods. In: Beynon RJ, Bond JS (eds) Proteolytic enzymes, a practical approach. Oxford, New York, Tokyo: IRL Press, pp 25–54
Shah DB, Wilson JB (1965) Egg yolk factor of Staphylococcus aureus. J Bacteriol 89:949–953
Talon R, Montel MC, Gandemer G, Viau M, Cantonnet M (1992) Lipolysis of pork fat by Staphylococcus warneri, S. saprophyticus and Micrococcus varians. Appl Microbiol Biotechnol 38:606–609
Tyski S, Hryniewicz W, Jeljaszewicz J (1983) Purification and some properties of the staphylococcal extracellular lipase. Biochim Biophys Acta 749:312–317
Vadehra DV (1974) Staphylococcal lipases. Lipids 9:158–165
Van Oort MG, Deveer AMTJ, Dijkman R, Tjeenk ML, Verheij HM, De Haas GH, Wenzig E, Götz F (1989) Purification and substrate specificity of Staphylococcus hyicus lipase. Biochemistry 28:9278–9285
Vogel RF, Gaier W, Hammes WP (1990) Expression of the lipase gene from Staphylococcus hyicus in Lactobacillus curvatus Lc2-c. FEMS Microbiol Lett 69:289–292
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Talòn, R., Dublet, N., Montel, MC. et al. Purification and characterization of extracellular Staphylococcus warneri lipase. Current Microbiology 30, 11–16 (1995). https://doi.org/10.1007/BF00294517
Issue Date:
DOI: https://doi.org/10.1007/BF00294517