Summary
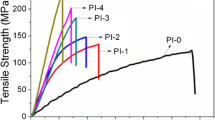
Imide-aryl ether benzothiazole random copolymers were investigated. A new diamine containing preformed benzothiazole rings was prepared by a thiazole activated nucleophilic aromatic substitution reaction. The synthesis involved the reaction of 2,6-(bis(4-fluorophenyl))benzo[1,2,4,5]bisthiazole with 3-aminophenol in the presence of K 2 CO 3 in N-methylprrolidone (NMP) to afford bis(3-aminophenoxy)phenylbenzo [1,2,4,5]bisthiazole in high yield. This new diamine was readily purified and co-reacted with various compositions of 4,4′-oxydianiline (ODA) and pyromellitic dianhydride (PMDA) producing a series of imide-ary ether benzothiazole random copolymers. Films were cast and cured (350°C) to effect imidization, affording tough films with moduli significantly higher than PMDA/ODA polyimide. The thermal stability of the copolymers was good with thermal decomposition temperatures in the 480 to 510°C range, and the thermal expansion coefficients were lower than PMDA/ODA polymide and in the 25–30 ppm range.
Similar content being viewed by others
References
Russell T P (1986) Polym Sci Polym Phys Ed 22: 1105
Takahashi N, Yoon D Y and Parrish W (1984) Macromolecules 17: 2583
Hedrick J L, Labadie J W, Russell T P (1989) Polyimides: Materials, Chemistry and Characterization, Feger C, Khojasteh M M and McGrath J E (eds.). Elsevier Science Publishers, Amsterdam, p. 61
Hedrick J L, Hilborn J, Labadie J, Russell T P (1990) Polymer: 000
Preston J, Dewinter W F, Black W B (1969) J Polym Sci A-1, 7: 283
Preston J, Dewinter W F, Black W B, Hofferbert W L, Jr (1969) J Polym Sci A-1, 7: 3027
Preston J (1965) Heterocyclic Chem 2: 441
Hedrick J L, Labadie J W (1990) Macromolecules 23: 1561
Hilborn J, Labadie J W, Hedrick J L (1990) Macromolecules: 000
Wolfe J F (1989) EPST 11: 601
Helminiak T E, Arnold F E, Benner C L (1975) Polym Preprints 16(2): 659
Volksen W, Cotts P M (1982) Polyimides: Synthesis, Characterization and Applications, Mittal K L (ed.). Plenum Press, New York, p. 163
Kawakami J H, Kwiatkowski G T, Brode G L, Bedwin, A W (1974) J Polym Sci, Plym Chem Ed. 12: 565
Hergenrother P M, Wakelyn N T, Havens S J (1987) Polym Preprints 28(1): 92
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hedrick, J.L. Imide-aryl ether benzothiazole copolymers. Polymer Bulletin 24, 371–377 (1990). https://doi.org/10.1007/BF00294089
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294089