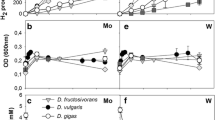
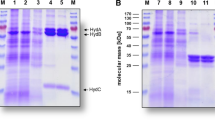
Abstract
Enzyme measurements were carried out with crude cell-free extracts of the propionate oxidizing coculture of Syntrophobacter wolinii and Desulfovibrio G11. Using cell-free extracts of a pure culture of Desulfovibrio G11 as a blank, most of the enzymes involved in the methylmalonyl-CoA pathway for propionate oxidation, including a propionyl-CoA: oxaloacetate transcarboxylase, were demonstrated in S. wolinii.
Similar content being viewed by others
References
Bergsma J, Dongen MBM van, Konings WN (1982) Purification and characterization of NADH dehydrogenase from Bacillus subtilis. Eur J Biochem 128:151–157
Boone DR, Bryant MP (1980) Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol 40:626–632
Boone DR, Johnson RL, Liu Y (1989) Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems, and applications in the measurement of KM for H2 and formate uptake. Appl Environ Microbiol 55:1735–1741
Boonstra J, Huttunen MT, Konings WN (1975) Anaerobic transport in Escherichia coli membrane vesicles. J Biol Chem 250:6792–6798
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bryant MP (1979) Microbial methane production — theoretical aspects. J Anim Sci 48:193–201
Buswell AM, Fina L, Müller H, Yahiro A (1951) Use of C14 in mechanism studies of methane fermentation. II. Propionic acid. J Am Chem Soc 73:1809–1811
Daniels L, Fuchs G, Thauer RK, Zeikus JG (1977) Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol 132:118–126
Dixon GH, Kornberg HL (1959) Assay methods for key enzymes of the glyoxylate cycle. Biochem J 72:3p
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Sci Tech 15:127–167
Hilpert W, Schink B, Dimroth P (1984) Life by a new decarboxylation-dependent energy conservation mechanism with Na+ as coupling ion. EMBO J 3:1665–1670
Houwen FP, Dijkema C, Schoenmakers CHH, Stams AJM, Zehnder AJB (1987) 13C-NMR study of propionate degradation by a methanogenic coculture. FEMS Microbiol Lett 41:269–274
Houwen FP, Cheng Guangsheng, Folkers GE, Heuvel WMJG van de, Dijkema C (1988) Pyruvate and fumarate conversion by a methanogenic propionate-oxidizing coculture. In: Lettinga G, Zehnder AJB, Grotenhuis JTC, Hulshoff Pol LW (eds) Granular anaerobic sludge; microbiology and technology. Pudoc, Wageningen, pp 62–70
Houwen FP, Plokker J, Dijkema C, Stams AJM (1990) Syntrophic propionate oxidation. In: Belaich JP, Bruschi M, Garcia JL (eds) Microbiology and biochemistry of strict anaerobes involved in interspecies hydrogn transfer. Plenum Press, New York, pp 281–289
Jngermann K, Schön G (1974) Pyruvate formate lyase in Rhodosprillium rubrum Ha adapted to anaerobic dark conditions. Arch Microbiol 99:109–116
Kaspar HF, Wuhrmann K (1978) Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol 36:1–7
Koch ME, Dolfing J, Wuhrmann K, Zehnder AJB (1983) Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol 45:1411–1414
Kremer DR, Hansen TA (1988) Pathway of propionate degradation in Desulfobulbus propionicus. FEMS Microbiol Lett 49:273–277
Maeba P, Sanwal BD (1969) Phosphoenolpyruvate carboxylase from Salmonella typhimurium, strain LT2. In: Lowenstein JM (ed) Methods in enzymology, vol 13. Academic Press, New York London, pp 283–288
Mah RA, Xun LY, Boone DR, Arhing B, Smith PH, Wilkie A (1990) Methanogenesis from propionate in sludge and enrichment cultures. In: Belaich JP, Bruschi M, Garcia JL (eds) Microbiology and biochemistry of strict anaerobes involved in interspecies hydrogen transfer. Plenum Press, New York, pp 99–111
Oberlies G, Fuchs G, Thauer RK (1980) Acetate thiokinase and the assimilation of acetate in Methanobacterium thermoautotrophicum. Arch Microbiol 128:248–252
Odom JM, Peck HD (1981) Localization of dehydrogenases, reductases, and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol 147:161–169
Pfennig N, Lippert KD (1966) Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol 55:245–256
Rabin R, Reeves HC, Wegener WS, Megraw RE, Ajl SJ (1965) Glyoxylate in fatty-acid metabolism. Condensations of glyoxylate with fatty acids lead to alternate pathways of fatty-acid metabolism. Science 150:1548–1558
Robbins JE (1988) A proposed pathway for catabolism of propionate in methanogenic cocultures. Appl Environ Microbiol 54:1300–1301
Schink B (1985) Mechanisms and kinetics of succinate and propionate degradation in anoxic freshwater sediments and sewage sludge. J Gen Microbiol 131:643–650
Scrutton MC, Olmsted MR, Utter MF (1969) Pyruvate carboxylase from chicken liver. In: Lowenstein JM (ed) Methods in enzymology, vol 13. Academic Press, New York London, pp 235–249
Stams AJM, Veenhuis M, Weenk GH, Hansen TA (1983) Occurence of polyglucose as a storage polymer in Desulfovibrio species and Desulfobulbus propionicus. Arch Microbiol 136:54–59
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA (1984) Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Wegener WS, Reeves HC, Rabin R, Ajl SJ (1968) Alternate pathways of metabolism of short-chain fatty acids. Bacteriol Rev 32:1–26
Zehnder AJB (1978) Ecology of methane formation. In: Mitchell R (ed) Water pollution microbiology, vol 2. Wiley, New York, pp 349–376
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Houwen, F.P., Plokker, J., Stams, A.J.M. et al. Enzymatic evidence for involvement of the methylmalonyl-CoA pathway in propionate oxidation by Syntrophobacter wolinii . Arch. Microbiol. 155, 52–55 (1990). https://doi.org/10.1007/BF00291274
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00291274