Summary
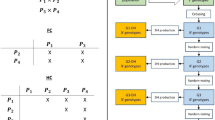
The genetic parameters of two quantitative traits, 13-day larval weight and pupal weight, in Tribolium populations developed by reciprocal recurrent selection (RRS) and by within-line purebred selection (WLS) were compared each with the other and also with the parameters of the unselected base populations using the genetic model of Carbonell, Nyquist and Bell. The variability for two and three-way crosses of inbred lines derived from “companion” populations (two strains, breeds, or varieties used for a terminal cross or hybrid) was analyzed into genetic effects: autosomal additivity (* g), autosomal heterosis (* s), sex-linked additivity (L), sex-linked heterosis (LL), general maternal (m), specific maternal or reciprocal (r), additive by additive epistasis (aa), and deviations from the model due, among other causes, to higher order epistasis (dev). One series of crosses involved companion populations with diverse origins. For contrast, a second series of crosses involved companion populations originating from a common heterogenous base population. For the heterotic trait larval weight, * g and * s effects were equally important and accounted for over 50% of the total variation. The aa epistasis contributed another 20% and was followed in importance by higher order epistasis and general maternal effects. For the more highly heritable trait, pupal weight, * g effects were most important with * s, aa, and m effects having smaller but significant influences. Sex-linked and reciprocal effects were statistically significant for many crosses, but they were relatively unimportant overall. In general, the unselected base populations showed higher * g variation than either RRS or WLS populations with the reverse true for * s effects. In agreement with theoretical expectations, RRS was more effective than WLS in exploiting * s effects. The aa epistatic effects for larval weight were of major importance in the unselected populations, but RRS and WLS did not differ significantly for exploiting superior aa gene combinations. Companion populations with diverse origins revealed significantly larger variation due to * g and * s effects in crosses than did populations initiated from a common heterogeneous base.
Similar content being viewed by others
References
Anderson VL (1970) Restriction errors for linear models. (An aid to develop models for designed experiments.) Biometrics 26:255–268
Bell AE (1969) The nature of selection responses in Tribolium. Jpn J Genet 44:299–309
Bell AE (1981) On the utilization of total genetic variation, an overview. Génétique quantitative et appliquée des populations croissées, Toulouse. Colloq INRA 10:107–123
Bell AE (1982 a) The Tribolium model in animal breeding research. Proc 2nd World Congr Genet Appl Livestock Prod 5:26–42
Bell AE (1982 b) Selection for heterosis, results with laboratory and domestic animals. Proc 2nd World Congr Genet Appl Livestock Prod 6:206–227
Bell AE, Moore CH (1972) Reciprocal recurrent selection for pupal weight in Tribolium in comparison with conventional methods. Egypt J Genet Cytol 1:92–119
Bell AE, Moore CH, Bohren BB, Warren DC (1952) Systems of breeding designed to utilize heterosis in the domestic fowl. Poult Sci 31:11–22
Bradley RA, Schumann DEW (1956) The comparison of the sensitivities of similar experiments: Applications. Biometrics 13:496–510
Brown WP, Bell AE (1980) An experimental comparison of selection alternatives to plateaued responses. Genetics 94:477–496
Carbonell EA, Nyquist WE, Bell AE (1983) Sex-linked and maternal effects in the Eberhart-Gardner general genetics model. Biometrics 39:607–619
Carbonell EA, Frey JJ, Bell AE (1985) Estimation of maternal, sex-linked and additive x additive epistatic gene effects for body size of Tribolium. Theor Appl Genet 70:133–137
Comstock RE, Robinson HF, Harvey PH (1949) A breeding procedure designed to make maximum use of both general and specific combining ability. Agron J 41:360–367
Costantino RF, Bell AE, Rogler JC (1967) Genetic analysis of a population of Tribolium. 1. Corn oil sensitivity and selection response. Heredity 22:529–539
Dearborn DD, Gregory KE, Cundiff LV, Koch RM (1987) Maternal heterosis and grandmaternal effects in beef cattle: Preweaning traits. J Anim Sci 65:33–41
Dickerson GE (1969) Experimental approaches in utilizing breed resources. Anim Breed Abstr 37:191–202
Eberhart SA, Gardner CO (1966) A general model for genetic effects. Biometrics 22:864–881
Eisen EJ, Hortsgen-Schwark G, Bandy TR, Saxton AM (1985) Diallel cross among lines of mice selected for litter size and body weights: Body composition traits. Z Tierz Züchtungsbiol 102:10–22
Kinghorn B (1982) Genetic effects in crossbreeding. 1. Models of merit. Z Tierz Züchtungsbiol 99:59–68
Kinghorn B (1983) Genetic effects in crossbreeding. 3. Epistatic loss in crossbred mice. Z Tierz Züchtungsbiol 100:209–222
Kinghorn B (1987) The nature of 2-locus epistatic interactions in animals: evidence from Sewal Wright's guinea pig data. Theor Appl Genet 73:595–604
McNew RW, Bell AE (1971) The nature of the purebred-crossbred genetic covariance. Genet Res 18:1–7
McNew RW, Bell AE (1974) Crossbred response from purebred selection, and experimental check on selection theory with Tribolium. Theor Appl Genet 44:100–105
McNew RW, Bell AE (1976) Comparison of cross bred and purebred selection for a heterotic trait in highly selected populations of Tribolium. J Hered 67:275–283
Melchinger AE, Geiger HH, Schnell FW (1986) Epistasis in maize (Zea mays L.). 2. Genetic effects in crosses among early flint and dent inbred lines determined by three methods. Theor Appl Genet 72:231–239
Orozco F, Bell AE (1974) Reciprocal recurrent selection compared to within-strain selection for increasing rate of egg lay of Tribolium under optimal and stress conditions. Genetics 77:143–161
Rich SS, Bell AE (1980) Genotypic-environment interaction effects in long-term selected populations of Tribolium. J Hered 71:319–322
Schumann DEW, Bradley RA (1959) The comparison of the sensitivities of similar experiments: Model II of the analysis of variance. Biometrics 15:405–416
Sheridan AK (1980) A new explanation for egg production in crosses between White Leghorns and Australorps. Br Poult Sci 21:85–88
Stuber CW, Moll RH (1971) Epistasis in maize (Zea mays L.). 2. Comparison of selected with unselected populations. Genetics 67:137–149
Author information
Authors and Affiliations
Additional information
Communicated by J. S. F. Barker
Journal Paper No. 11559 from Purdue University Agricultural Experimental Station
Rights and permissions
About this article
Cite this article
Carbonell, E.A., Bell, A.E. & Frey, J.J. Non-additive gene effects in populations under different methods of selection. Theoret. Appl. Genetics 78, 567–580 (1989). https://doi.org/10.1007/BF00290844
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00290844