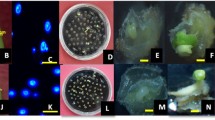
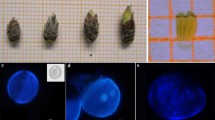
Summary
A direct comparison of microspore culture and anther culture was made in Brassica napus using F1 crosses of Regent (canola) by Golden (rapeseed), and their reciprocals, as well as a hybrid between Reston and a highly embryogenic, canola-quality breeding line (G231) as donor plants. The study confirmed that microspore culture can be ten times more efficient than anther culture for embryo production. Embryo yields from cultures initiated from the Reston x G231 were four-fold greater than those initiated from the Regent x Golden crosses, and significant differences were also detected among cultures initiated from the different Regent x Golden crosses. These results illustrate the influence that donor plant genotype has on embryo production. However, superior embryogenic potential among donor material was not always coincident with superior plant production. The average haploid-todiploid ratio in microspore-derived regenerates was 2∶1 for the population obtained from the Regent x Golden crosses but 1∶1 for the Reston x G231 cross. For both types of material, the frequency of diploids increased upon repeated cycles of explanting. A field study showed that there were no differences between the populations of anther-derived and microspore-derived spontaneous diploid and doubled haploid lines, with respect to the days required for them to flower or to mature. The information is valuable for canola breeding programs considering the use of haploidy.
Similar content being viewed by others
References
Chen J, Beversdorf WD (1987) Comparison of fatty acid variation in spring rapeseed (B. napus) derived from microspore culture and single seed descent. Proc Crucifer Genet Workshop IV, University of Wisconsin, Madison
Chuong PV, Beversdorf WD (1985) High frequency embryogenesis through isolated microspore culture in Brassica napus L. and Brassica carinata Braun. Plant Sci 39:219–226
Chuong PV, Deslauriers C, Kott LS, Beversdorf WD (1988a) Effects of donor genotype and bud sampling in microspore culture of Brassica napus. Can J Bot 66:1653–1657
Chuong PV, Pauls KP, Beversdorf WD (1988b) High frequency embryogenesis in male sterile plants of Brassica napus through microspore culture. Can J Bot 66:1676–1680
Downey RK, Harvey BL (1963) Method of breeding for oil quality in rape. Can J Plant Sci 43:271–275
Dunwell JM, Cornish M (1985) Influence of preculture variables on microspore embryo production in Brassica napus ssp. oleifera cv Duplo. Ann Bot 56:281–289
Dunwell JM, Cornish M, Courcel AGL de (1985) Influence of genotype, plant growth temperature and anther incubation temperature on microspore embryo production in Brassica napus ssp. oleifera. J. Exp Bot 36:679–689
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Guha S, Iyer RD, Gupta N, Swaminathan MS (1970) Totipotency of gametic cells and the production of haploids in rice. Curr Sci 39:174–176
Heberle-Bors E (1984) Genotypic control of pollen plant formation in Nicotiana tabacum L. Theor Appl Genet 68:475–479
Hoffmann F (1980) Pflanzliche Zellkulturtechniken als Züchtungsschritt am Beispiel Raps. Naturwissenschaften 67: 301–306
Hoffmann F, Thomas E, Wenzel G (1982) Anther culture as a breeding tool in rape. 2. Progeny analyses of androgenetic lines and induced mutants from haploid cultures. Theor Appl Genet 61:225–232
Keller WA (1984) Anther culture of Brassica. In: IK Vasil (ed) Cell culture and somatic cell genetics of plants, vol 1. Academic Press, New York, pp 302–310
Keller WA, Armstrong KC (1978) High frequency production of microspore-derived plants from Brassica napus anther culture. Z Pflanzenzuecht 80:100–108
Keller WA, Armstrong KC (1979) Stimulation of embryogenesis and haploid production in Brassica campestris anther culture by elevated temperature treatments. Theor Appl Genet 55:65–67
Keller WA, Armstrong KC (1983) Production of Brassica napus haploids through anther and microspore culture. Proc 6th Int Rapeseed Conf, Paris
Keller WA, Stringam GR (1978) Production and utilization of microspore-derived plants from Brassica napus anther culture. In: TA Thorpe (ed) Proc IAPTC: Frontiers of plant tissue culture, International Association for Plant Tissue Culture, University of Calgari, pp 113–122
Keller WA, Rajhathy T, Lacapra J (1975) In vitro production of plants from pollen in Brassica campestris. Can J Genet Cytol 17:655–666
Keller WA, Armstrong KC, Roche AI de la (1982) The production and utilization of microspore-derived haploids in Brassica crops. In: SK Sens; KL Giles (eds) Plant cell culture in crop improvement. Plenum Press, New York, pp 169–183
Kott LS, Polsoni L, Beversdorf WD (1988) Pre-and postmitotic cytological evens in isolated microspore culture of Brassica napus. Can J Bot 66:1658–1664
Lichter R (1981) Anther culture of Brassica napus in liquid culture medium. Z Pflanzenphysiol 103:229–237
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol 105:427–434
Lichter R, Groot E de, Fiebig D, Schweiger R, Gland A (1988) Glucosinolates determined by HPLC in seeds of microspore-derived lines of rapeseed (Brassica napus L.). Plant Breed 100:201–221
Nitsch JP (1969) Experimental androgenesis in Nicotiana. Phytomorphology 19:389–404
Nitsch C, Nitsch JP (1967) The induction of flowering in vitro in stem segments of Plumbago indica L. I. The production of vegetative buds. Planta 72:355–370
Sacristan MD (1971) Karyotypic changes in callus cultures from haploid and diploid plants of Crepis capillarii. Chromosoma 33:273–283
Sequin-Schwartz G, Hutcheson DS, Downey RK (1983) Anther culture studies in Brassica campestris. Proc 6th Int Rapeseed Conf, Paris
Siebel J, Pauls KP (1987) The use of haploids in Brassica napus breeding. Proc Crucifer Genet Workshop IV, University of Wisconsin, Madison
Swanson EB, Coumans MP, Wu SC, Barsby TL, Beversdorf WD (1987) Efficient isolation of microspores and the production of microspore-derived embryos from Brassica napus. Plant Cell Rep 6:94–97
Thomas E, Wenzel G (1975) Embryogenesis from microspores of Brassica napus. Z Pflanzenzuecht 74:77–81
Thurling N, Das LDV (1979) The influence of donor plant genotype and environment on the production of multicellular microspores in cultures of anthers of Brassica napus ssp. oleifera. Ann Bot 54:681–693
Wenzel G (1979) Recent progress in microspore culture of crop plants. In: Davies DR, Hopwood DA (eds) The plant genome. The John Innes Charity, Norwich
Author information
Authors and Affiliations
Additional information
Communicated by G. Wenzel
Rights and permissions
About this article
Cite this article
Siebel, J., Pauls, K.P. A comparison of anther and microspore culture as a breeding tool in Brassica napus . Theoret. Appl. Genetics 78, 473–479 (1989). https://doi.org/10.1007/BF00290830
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00290830