Summary
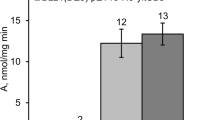
The structural gene for the Bacillus stearothermophilus glycogen branching enzyme (glgB) was cloned in Escherichia coli. Nucleotide sequence analysis revealed a 1917 nucleotide open reading frame (ORF) encoding a protein with an Mr of 74787 showing extensive similarity to other bacterial branching enzymes, but with a shorter N-terminal region. A second ORF of 951 nucleotides encoding a 36971 Da protein started upstream of the glgB gene. The N-terminus of the ORF2 gene product had similarity to the Alcaligenes eutrophus czcD gene, which is involved in cobalt-zinc-cadmium resistance. The B. stearothermophilus glgB gene was preceded by a sequence with extensive similarity to promoters recognized by Bacillus subtilis RNA polymerase containing sigma factor H (E - σH). The glgB promoter was utilized in B. subtilis exclusively in the stationary phase, and only transcribed at low levels in B. subtilis spoOH, indicating that sigma factor H was essential for the expression of the glgB gene in B. subtilis. In an expression vector, the B. stearothermophilus glgB gene directed the synthesis of a thermostable branching enzyme in E. coli as well as in B. subtilis, with optimal branching activity at 53° C.
Similar content being viewed by others
References
Argos P, Rossmann MG, Grau UM, Zuber H, Frank G, Tratschin JD (1979) Thermal stability and protein structure. Biochemistry 18:5698–5703
Baecker PA, Greenberg E, Preiss J (1986) Biosynthesis of bacterial glycogen: primary structure of Escherichia coli 1,4-α-d-glu-can:1,4-α-d-glucan 6-α-d-(1,4-α-d-glucano)-transferase as deduced from the nucleotide sequence of the glgB gene. J Biol Chem 261:8738–8743
Band L, Henner DJ (1984) Bacillus subtilis requires a “stringent” Shine-Dalgarno region for gene expression. DNA 3:17–21
Biggin MD, Gibson TJ, Hong GF (1983) Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci USA 80:3963–3965
Bowen D, Littlechild JA, Fothergill JE, Watson HC, Hall L (1988) Nucleotide sequence of the phosphoglycerate kinase gene from the extreme thermophile Thermus thermophilus: comparison of the deduced amino acid sequence with that of the mesophilic yeast phosphoglycerate kinase. Biochem J 254:509–517
Boyer C, Preiss J (1977) Biosynthesis of bacterial glycogen: purification and properties of the Escherichia coli B α-1,4-glucan:α-1,4-glucan 6-glycosyltransferase. Biochemistry 16:3693–3699
Bron S, Venema G (1972) Ultraviolet inactivation and excision repair in Bacillus subtilis: I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet sensitive derivatives. Mutat Res 15:1–10
Brosius J (1984) Toxicity of an overproduced foreign gene in Escherichia coli and its use in plasmid vectors for the selection of transcription terminators. Gene 27:161–172
Doi RH, Wang L-F (1986) Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev 50:227–243
Goldemberg SH (1972) Glucan biosynthesis in Bacillus stearothermophilus. 1. Properties of the polysaccharide. Arch Biochem Biophys 149:252–258
Hager PW, Rabinowitz JC (1985) Translational specificity in Bacillus subtilis. In: Dubnau D (ed) The molecular biology of the bacilli, vol 2. Academic Press, New York, pp 1–31
Haima P, Bron S, Venema G (1987) The effect of restriction on shotgun cloning and plasmid stability in Bacillus subtilis Marburg. Mol Gen Genet 209:335–342
Igo M, Lampe M, Losick R (1988) Structure and regulation of a Bacillus subtilis gene that is transcribed by the EσB form of RNA polymerase holoenzyme. In: Ganesan AT, Hoch JA (eds) Genetics and biotechnology of bacilli, vol 2. Academic Press, New York, pp 151–156
Ish-Horowicz D, Burke JF (1981) Rapid and efficient cosmid cloning. Nucleic Acids Res 9:2989–2998
Kiel JAKW, Vossen JPMJ, Venema G (1987) A general method for the construction of Escherichia coli mutants by homologous recombination and plasmid segregation. Mol Gen Genet 207:294–301
Kiel JAKW, Elgersma HSA, Beldman G, Vossen JPMJ, Venema G (1989) Cloning and expression of the branching enzyme gene (glgB) from the cyanobacterium Synechococcus sp. PCC7942 in Escherichia coli. Gene 78:9–17
Kiel JAKW, Boels JM, Beldman G, Venema G (1990) Nucleotide sequence of the Synechococcus sp. PCC7942 branching enzyme gene (glgB); expression in Bacillus subtilis. Gene 89:77–84
Kubo M, Imanaka T (1988) Cloning and nucleotide sequence of the highly thermostable neutral protease from Bacillus stearothermophilus. J Gen Microbiol 134:1883–1892
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Losick R, Youngman P, Piggot PJ (1986) Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet 20:625–669
Maniatis T, Fritsch EF, Sambrook F (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Messing J (1979) A multipurpose cloning system based on the single-stranded DNA bacteriophage M13. Recombinant DNA Technical Bulletin, NIH publication No. 79–99, vol 2, no 2, pp 43–48
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Nies DH, Nies A, Chu L, Silver S (1989) Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc Natl Acad Sci USA 86:7351–7355
Norrander J, Kempe T, Messing J (1983) Construction of improved M13 vectors using oligo deoxynucleotide-directed mutagenesis. Gene 26:101–106
Okita TW, Rodriguez RL, Preiss J (1981) Biosynthesis of bacterial glycogen: cloning of the glycogen biosynthetic enzyme structural genes of Escherichia coli. J Biol Chem 256:6944–6952
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Platt T (1986) Transcription termination and the regulation of gene expression. Annu Rev Biochem 55:339–372
Preiss J (1984) Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol 38:419–458
Preiss J, Romeo T (1989) Physiology, biochemistry and genetics of bacterial glycogen synthesis. In: Rose AH, Tempest DW (eds) Adv Microb Physiol 30:183–238
Qi F-X, Doi RH (1990) Localization of a second SigH promoter in the Bacillus subtilis sigA operon and regulation of dnaE expression by the promoter. J Bacteriol 172:5631–5636
Queen C, Korn LJ (1984) A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res 12:581–599
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Shields DC, Sharp PM (1987) Synonymous codon usage in Bacillus subtilis reflects both translational selection and mutational biases. Nucleic Acids Res 15:8023–8039
Silhavy TJ, Berman ML, Enquist LW (1984) Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Slock JA, Stahly DP (1974) Polysaccharide that may serve as a carbon and energy storage compound for sporulation in Bacillus cereus. J Bacteriol 120:399–406
Takagi M, Imanaka T, Aiba S (1985) Nucleotide sequence and promoter region for the neutral protease gene from Bacillus stearothermophilus. J Bacteriol 163:824–831
Tatti KM, Carter III HL, Moir A, Moran CP Jr (1989) Sigma H directed transcription of citG in Bacillus subtilis. J Bacteriol 171:5928–5932
Tinoco I Jr, Borer PN, Dengler B, Devine MD, Uhlenbeck OC, Crothers DM, Gralla J (1973) Improved estimation of secondary structure in ribonucleic acids. Nature 246:40–41
Tolmansky DS, Krisman CR (1987) The degree of branching in (α1,4)-(α1,6)-linked glucopolysaccharides is dependent on intrinsic properties of the branching enzymes. Eur J Biochem 168:393–397
Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Weber M, Wöber G (1975) The fine structure of the branched α-d-glucan from the blue-green alga Anacystis nidulans: comparison with other bacterial glycogens and phytoglycogen. Carbohydr Res 39:295–302
Weir J, Dubnau E, Ramakrishna N, Smith I (1984) Bacillus subtilis spoOH gene. J Bacteriol 157:405–412
Williams JM, Duvall EJ, Lovett PS (1981) Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol 146:1162–1165
Zulli F, Weber H, Zuber H (1987) Structure and function of l-lactate dehydrogenases from thermophilic and mesophilic bacteria, VI. Nucleotide sequences of lactate dehydrogenase genes from the thermophilic bacteria Bacillus stearothermophilus, B. caldolyticus and B. caldotenax. Biol Chem Hoppe-Seyler 368:1167–1177
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kiel, J.A.K.W., Boels, J.M., Beldman, G. et al. Molecular cloning and nucleotide sequence of the glycogen branching enzyme gene (glgB) from Bacillus stearothermophilus and expression in Escherichia coli and Bacillus subtilis . Molec. Gen. Genet. 230, 136–144 (1991). https://doi.org/10.1007/BF00290661
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00290661