Abstract
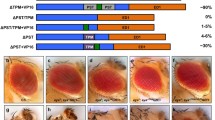
Analysis of the white zeste mottled (wzm) mutant family suggests that the zeste gene product functions in establishing and stabilizing a transcriptionally active chromatin domain for white locus expression. The z 1 mutation reduces expression of paired or proximate copies of white, while single or unpaired copies maintain wildtype levels of expression. The w zmmutation, caused by the insertion of the retrotransposon BEL into the 5′ intron of white, alters the zeste-white interaction to produce a mottled eye phenotype in hemizygous z 1 w zm males. We have determined the molecular structure of four w zmderivatives. w zlresults from the insertion of an additional transposable element into the 5′ regulatory region of white. w zvlis a deletion of sequences upstream of the white locus. Two others, w haloand w cres, result from the transposition of w zmplus the entire verticals-roughest region into heterochromatin near the tip of chromosome 3L. They variegate for roughest but not for white; rather, the z 1 effect on w zmnow causes white expression to become non-autonomous and non-clonal. The analysis of these five mutations shows that the neomorphic zeste 1 product, in combination with structural changes imposed by transposons and intercalary heterochromatin, modifies the determination and stability of white expression. We propose that the normal zeste product functions as part of a complex that stimulates transcription by changing chromatin conformation to establish and maintain transcriptionally active domains. The unpairing of homologs is proposed to be one of the initial results of conformational change, providing an explanation for the role of zeste in transvection.
Similar content being viewed by others
References
Ashburner MA (1989) Transvection effects. Drosophila: A laboratory handbook, Cold Spring Harbor Laboratory Press, NY, pp 916–929
Becker HJ (1959) New mutants. Dros Inf Serv 33:82
Becker HJ (1960) Variegation in the zeste eye color and its bearing on gene action during the development of the eye of Drosophila melanogaster. Genetics 45:519–534
Bell JR, Bogardus AM, Schmidt T, Pellegrini M (1985) A new copia-like transposable element found in Drosophila rDNA gene unit. Nucleic Acids Res 13:3861–3871
Benson M, Pirrotta V (1987) The product of the Drosophila zeste gene binds to specific DNA sequences in white and Ubx. EMBO J 6:1387–1392
Benson M, Pirrotta V (1988) The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: implications for transvection and gene regulation. EMBO J 7:3907–3915
Biggin MD, Bickel S, Benson M, Pirrotta V, Tjian R (1988) Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell 53:713–722
Bingham PM, Zachar Z (1985) Evidence that two mutations wDZL and z 1 affecting synapsis-dependent genetic behavior of white are transcriptional regulatory mutations. Cell 40:819–829
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Cusi MG, Ciob L, Rovera G (1992) PCR amplification of GC-rich templates containing palindromic sequences using initial alkali denaturation. BioTechniques 12:502–504
Gans M (1953) Etude génétique et physiologique du mutant z de Drosophila melanogaster. Bull Biol France Belgique 38:1–90
Gelbart WM (1982) Synapsis-dependent allelic complementation at the decapentoplegic gene complex in Drosophila melanogaster. Proc Natl Acad Sci USA 79:2636–2640
Gelbart WM, Wu C-T (1982) Interactions of zeste mutations with loci exhibiting transvection effects in Drosophila melanogaster. Genetics 102:179–189
Geyer PK, Green MM, Corces VG (1990) Tissue-specific transcriptional enhancers may act in trans on the gene located in the homologous chromosome: the molecular basis of transvection in Drosophila. EMBO J 9:2247–2256
Green MM (1967) The genetics of a mutable gene at the white locus of Drosophila melanogaster. Genetics 56:467–482
Hazelrigg T, Petersen S (1992) An unusual genomic position effect on Drosophila white gene expression: pairing dependence, interactions with zeste, and molecular analysis of revertants. Genetics 130:125–138
Jack JW, Judd BH (1979) Allelic pairing and gene regulation: a model for the zeste-white interaction in Drosophila melanogaster. Proc Natl Acad Sci USA 76:1368–1372
Johnson-Schlitz D, Lim JK (1987) Cytogenetics of Notch mutations arising in the unstable X chromosome Uc of Drosophila melanogaster. Genetics 115:701–709
Jones RS, Gelbart WM (1990) Genetic analysis of the Enhancer of zeste locus and its role in gene regulation in Drosophila melanogaster. Genetics 126:185–199
Judd BH (1963) The genetic fine structure of the mutants zm and zl in Drosophila melanogaster. In: Geerts SJ (ed) Genetics Today. Procedings XIth International Congress Genetics, vol 1. Pergamon Press, New York
Judd BH (1988) Transvection: allelic cross talk. Cell 53:841–843
Kassis JA, Van Sickle EP, Sensabaugh SM (1991) A fragment of engrailed regulatory DNA can mediate transvection of the white gene in Drosophila. Genetics 128:751–761
Levis R, Bingham PM, Rubin GM (1982) Physical map of the white locus of Drosophila melanogaster. Proc Natl Acad Sci USA 79:564–568
Levis R, Hazelrigg T, Rubin GM (1985) Separable cis-acting control elements for expression of the white gene of Drosophila. EMBO J 4:3489–3499
Lewis EB (1954) The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am Nat 88:225–239
Lindsley DL, Zimm GG (1992) The genome of Drosophila melanogaster. Academic Press, NY
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, NY
Mansukhani A, Crickmore A, Sherwood PW, Goldberg ML (1988) DNA-binding properties of the Drosophila melanogaster zeste gene product. Mol Cell Biol 8: 615–623
O'Hare K, Murphy C, Levis R, Rubin G (1984) DNA sequence of the white locus of Drosophila melanogaster. J Mol Biol 180:437–455
Paro R (1990) Imprinting a determined state into the chromatin of Drosophila. Trends Genet 6:416–421
Peterson KM (1990) The w zmmutation and its derivatives in Drosophila melanogaster. Ph. D. dissertation, University of North Carolina at Chapel Hill
Pirrotta V (1988) Vectors for P-mediated transformation in Drosophila. In: Rodriguez RL, Denhardt DT (ed) Vectors. A survey of molecular cloning vectors and their uses. Butterworths, Boston, pp 437–445
Pirrotta V (1991) The genetics and molecular biology of zeste in Drosophila melanogaster. Adv Genet 29: 301–348
Pirrotta V, Steller H, Bozzetti MP (1985) Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J 4:3501–3508
Qian S, Varjavand B, Pirrotta V (1992) Molecular analysis of the zeste-white interaction reveals a promoter-proximal element essential for distant enhancer-promoter communication. Genetics 131:79–90
Smolik-Utlaut SM, Gelbart WM (1987) The effects of chromosomal rearrangements on the zeste-white interaction in Drosophila melanogaster. Genetics 116:285–298
Tartof KD, Hobbs CA (1988) New cloning vectors and techniques that make restriction mapping easy. Gene 67:169–182
Wu C-T, Goldberg ML (1989) The Drosophila zeste gene and transvection. Trends Genet 5:189–194
Wu C-T, Jones RS, Lasko PF, Gelbart WM (1989) Homeosis and the interaction of zeste and white in Drosophila. Mol Gen Genet 218:559–564
Author information
Authors and Affiliations
Additional information
Communicated by M. Ashburner
Rights and permissions
About this article
Cite this article
Peterson, K.M., Davis, P.S. & Judd, B.H. The determined state of white expression in the Drosophila eye is modified by zeste 1in the w zm family of mutants. Molec. Gen. Genet. 242, 717–726 (1994). https://doi.org/10.1007/BF00283427
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00283427