Abstract
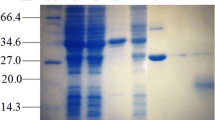
With a view to exploring its use as a metal-binding factor in transgenic plants we prepared the α-domain of metallothionein by reconstitution of rabbit apometallothionein and proteolysis of MT-1 and MT-2 with subtilisin. The isolated α-domains were characterised by UV and CD spectroscopy Double-Stranded. DNA encoding the a-domain (106 bp) of the human MTIA was constructed from chemically synthesized oligomers by repair synthesis and enzymatic ligation, cloned into pUC19 and sequenced. A expression construct containing the cloned α-domain was introduced into tobacco cells on a disarmed Agrobacterium tumefaciens Ti-plasmid. Transformed tobacco cells were selected and regenerated on medium containing cadmium and kanamycin. The growth of roots and shoots of transformants was unaffected by up to 100 μM cadmium, whereas control plants showed severe inhibition of root and shoot growth, and chlorosis of leaves on medium containing only 10 μM cadmium. Southern hybridization confirmed the presence of the transgene in the transformed plant tissues. The concentration of human α-domain peptides in transgenic tobacco eaves was determined by the Cd/hemoglobin saturation assay and polarography using the rabbit α-domain as standard. The results indicate that the α-domain, one of two domains in MT molecules, is not only stable in vitro, but is also expressed efficiently and functions independently in transgenic plant cells.
Similar content being viewed by others
References
Atkinson T, Smith M (1984) Solid-phase synthesis of oligodeoxyribonucleotides by the phosphite triester method. In: Gait MJ (ed) Oligonucleotide synthesis. A practical approach. IRL Press, Oxford, pp 35–81
Bevan MW (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721
Bremner I, Young BW (1976) Isolation of (Copper, Zinc) thioneins from the livers of copper-injected rats. Biochem J 157:517–520
Doyle JJ, Doyle JL (1988) Isolation of plant DNA from fresh tissue. BRL Focus 12:13–15
Höfgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16:9877
Horsch RB, Fraley RT, Rogers SG, SandersPR, Lloyd A, Hoffman N (1984) Inheritance of functional foreign genes in plants. Science 223:496–498
Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Kägi JHR, Schäffer A (1988) Biochemistry of metallothionein. Biochemistry 27:8509–8515
Karin M, Richards RI (1982) Human metallothionein genes — primary structure of the metallothionein — II gene and a related processed gene. Nature 299:797–802
Li TY, Kraker AJ, Shaw CF, Petering DH (1980) Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc Natl Acad Sci USA 77:6334–6338
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nielson KB, Winge DR (1983) Order of metal binding in metallothionein. J Biol Chem 258:13063–13069
Nielson KB, Winge DR (1985) Independence of the domains of metallothionein in metal binding. J Biol Chem 260:8698–8701
Onosaka S, Cherian MG (1982) Comparison of metallothionein determination by polarographic and cadmium — saturation methods. Toxic Appl Pharm 63:270–274
Sanders PR, Winters JA, Barnason AR, Rogers SG, Fraley RT (1987) Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res 15:1543–1558
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Sci USA 74:5463–5467
Varma MM, Katz HM (1978) Environmental impact of cadmium. J Environ Health 40:308–314
Winge DR, Miklossy KA (1982) Domain nature of metallothionein. J Biol Chem 257:3471–3476
Winge DR, Premakumar R, Rajagopalan KV (1975) Metal-induced formation of metallothionein in rat liver. Arch Biochem Biophys 170:242–252
Author information
Authors and Affiliations
Additional information
Communicated by R. Hagemann
Rights and permissions
About this article
Cite this article
Pan, A., Tie, F., Duau, Z. et al. α-Domain of human metallothionein IA can bind to metals in transgenic tobacco plants. Molec. Gen. Genet. 242, 666–674 (1994). https://doi.org/10.1007/BF00283421
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00283421